China’s NMPA accepts IND for SinoMab BioScience’s SM17 to treat asthma
Pharmaceutical Technology
MAY 22, 2023
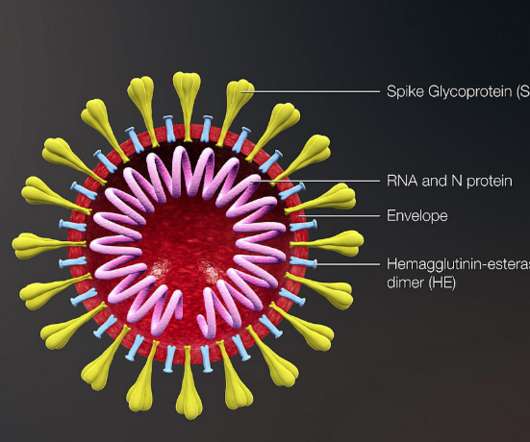
It can suppress Th2 immune responses by binding to IL-17RB on Type 2 innate lymphoid cells (ILC2s). This blocks a cascade of responses that are induced by interleukin-25 (IL-25), a critical cytokine which is classified as ‘alarmin’.






































Let's personalize your content