Why Should Companies Use Digital Endpoints Across Clinical Development?
XTalks
MAY 28, 2024
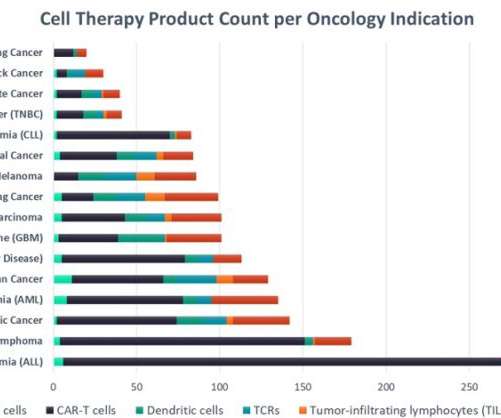
Phase III trials: In these advanced stages, focused on confirming efficacy and monitoring side effects in larger populations, DHTs may support market authorization and label claims. This approach can speed up development and improve data quality, potentially leading to more effective and personalized therapies.








































Let's personalize your content