Flow cytometry: advantages in immunotherapy clinical trials
Pharmaceutical Technology
AUGUST 24, 2022
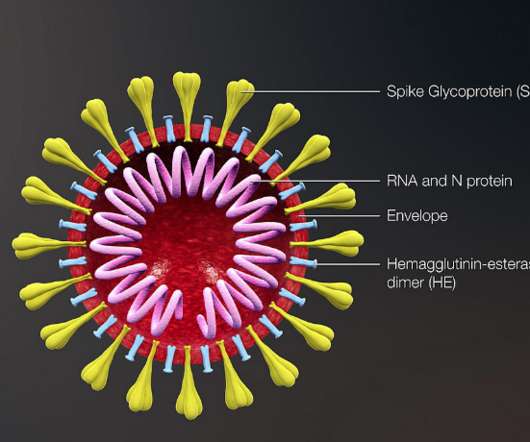
Patients provide blood samples for clinical trial sponsors to develop and deliver these emerging immunotherapies. A central lab with a global footprint provides both the efficient transportation arrangement and scientific rigor required to unlock the valuable information contained within patients’ biological samples.











































Let's personalize your content