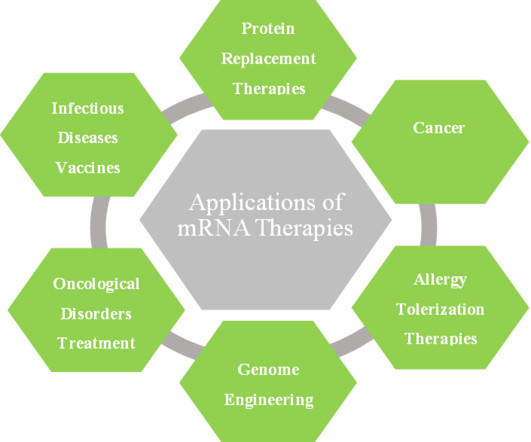
BioNTech shelves oral mRNA vaccine plans with Matinas
Pharmaceutical Technology
MAY 12, 2023
The in vivo study, conducted in mice, involved a phosphatidylserine-containing nano-formulation of mRNA supplied by BioNTech, distinct from traditional lipid nanocrystals (LNCs). Whilst the formulation had been successful in vitro , the oral administration in mice did not elicit activity.












































Let's personalize your content