AZD7442 reduced risk of developing severe COVID-19 or death in TACKLE Phase III outpatient treatment trial
The Pharma Data
OCTOBER 12, 2021
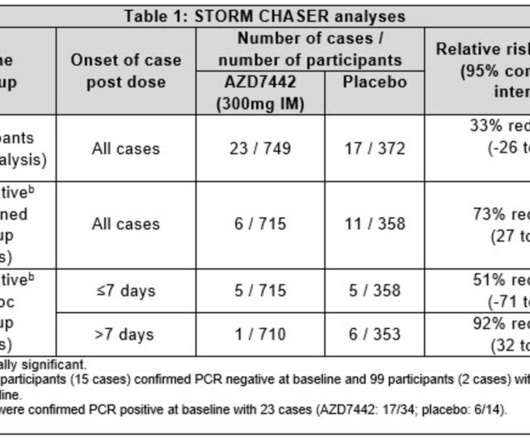
Trial met primary endpoint. The trial met the primary endpoint, with a cure of 600 mg of AZD7442 given by intramuscular (IM) injection reducing the threat of developing severe COVID-19 or death (from any cause) by 50 compared to placebo in rehabilitants who had been characteristic for seven days or lower.

















Let's personalize your content