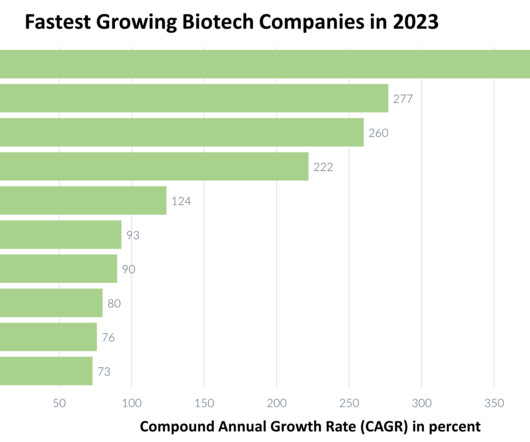
Touchlight boosts DNA manufacturing capacity with latest expansion
Pharmaceutical Technology
MAY 26, 2023
Enzymatic DNA production company Touchlight have augmented its DNA production capabilities with a newly announced expansion to its London facilities. Tripling its production capacity, Touchlight can now manufacture 8kg plasmid DNA, a key component for mRNA gene therapies and vaccines.































Let's personalize your content