Analytical method to simplify the characterization of membrane proteins
Drug Discovery World
DECEMBER 29, 2023
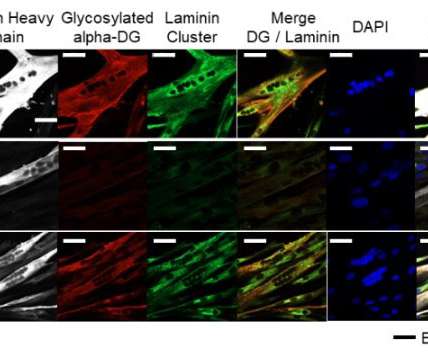
Dr Sofia Ferreira, Director of Applications at Refeyn, discusses how various technologies can address the main challenges in membrane protein characterization, describing how novel technologies, such as mass photometry, can streamline and support analytical processes. Scientists use a multitude of membrane mimetics to overcome this issue.




































Let's personalize your content