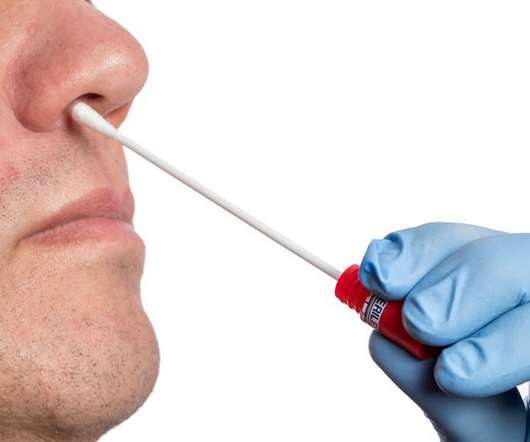
FDA Approves First Over-the-Counter Fully At-Home Test for COVID-19
The Pharma Data
DECEMBER 15, 2020
FDA Approves First Over-the-Counter Fully At-Home Test for COVID-19. Last week, the FDA approved a different at-home test, but it requires samples to be mailed to a lab to get the results. Last week, the FDA approved a different at-home test, but it requires samples to be mailed to a lab to get the results.














Let's personalize your content