2022 in review: Regulation starts to catch up with AI in pharma
Pharmaceutical Technology
DECEMBER 21, 2022
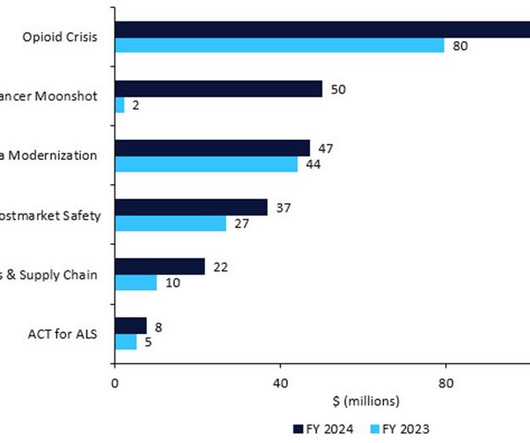
But as the field grows in leaps and bounds, many authorities have prioritised the release of new guidelines, frameworks, and regulations to keep pace with these advances. This could spell high-stake consequences for consumers whose privacy and safety could be at risk if AI models are not regulated. Major AI deals in 2022.













































Let's personalize your content