Mastering Responses to FDA 510(k) AI Letters: A Strategic Approach
FDA Law Blog
JULY 10, 2023
By Day 7 FDA sends Acknowledgment Letter. OR FDA sends Hold Letter if unresolved issues with User Fee and/or eCopy. If the submission passes the acceptance review, the firm will receive the first good news from FDA via email that the submission has been accepted for substantive review. By Day 15 FDA conducts Acceptance Review.



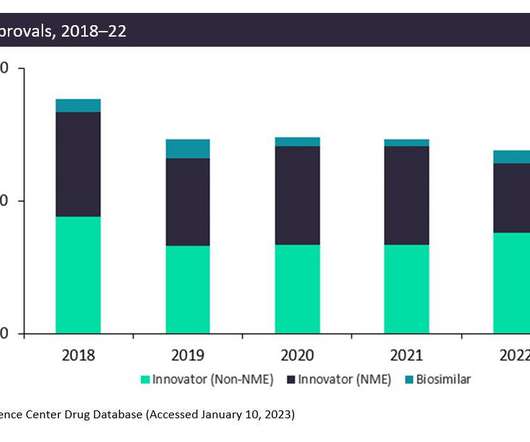









































Let's personalize your content