Cannabinoids receptors: popular preclinical target but banned in 137 countries
Pharmaceutical Technology
DECEMBER 5, 2022
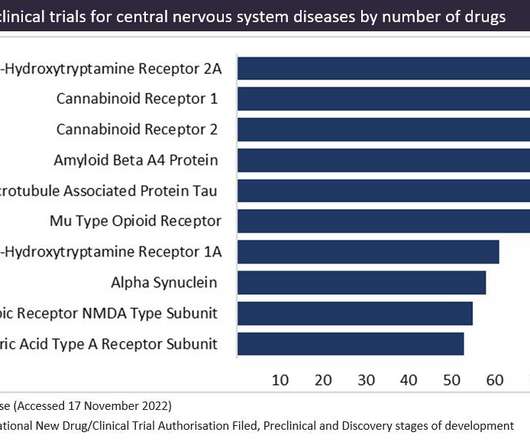
Cannabinoid receptors are a popular therapeutic target for cannabinoid-based drugs in the treatment of pain, neurological disorders and inflammation, according to GlobalData’s Pharma Intelligence Centre Drugs database. Cannabinoid-based drugs are derived from compounds found in the cannabis plant.


























Let's personalize your content