Sporting a $3.5M price tag, CSL and uniQure's hemophilia B gene therapy crosses FDA finish line
Fierce Pharma
NOVEMBER 23, 2022
price tag, CSL and uniQure's hemophilia B gene therapy crosses FDA finish line. Sporting a $3.5M Wed, 11/23/2022 - 10:10.

 tag hemophilia
tag hemophilia 
Fierce Pharma
NOVEMBER 23, 2022
price tag, CSL and uniQure's hemophilia B gene therapy crosses FDA finish line. Sporting a $3.5M Wed, 11/23/2022 - 10:10.

Bio Pharma Dive
AUGUST 25, 2022
On a conference call, BioMarin executives revealed the anticipated price for Roctavian and the company's initial launch plans, which include pay-for-performance deals customized to different markets.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
AUGUST 28, 2020
A draft report from the drug pricing watchdog concluded that, under the right conditions, Roctavian may be cost-effective at a price of $2.5

Medical Xpress
NOVEMBER 23, 2022
People with one form of the genetic blood disorder hemophilia now have a one-time treatment with a $3.5 million price tag.
Pharmaceutical Technology
FEBRUARY 10, 2023
On November 22, 2022, the FDA approved CSL Behring’s Hemgenix (etranacogene dezaparvovec), the first gene therapy treatment for hemophilia B, with a staggering manufacturer price of $3.5 This is not the first treatment to come with a high price tag.

XTalks
DECEMBER 13, 2023
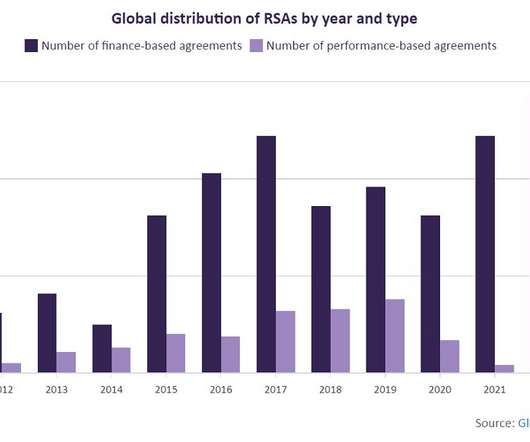
Related: Roctavian Becomes First Gene Therapy for Severe Hemophilia A to Get FDA Nod Casgevy and Lyfgenia Pricing and Warnings The price tags of both gene therapies are steep, given the complexity of developing gene therapies and as therapies meant to be one-time treatments.

XTalks
NOVEMBER 24, 2022
In a pivotal approval, the US Food and Drug Administration (FDA) has given the nod to a new gene therapy called Hemgenix (etranacogene dezaparvovecfor) for the treatment of adults with the genetic blood disorder hemophilia B (congenital Factor IX deficiency). Hemophilia B primarily affects men. With a list price of $3.5
Let's personalize your content