April 20, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Bioinformatic exploration of perivascular space discovers 24 genomic risk loci
An international team of 79 researchers have collaborated on a study published in Nature Medicine to delve into perivascular spaces (PVS), a poorly understood artifact seen in magnetic resonance imaging of cerebral small vessel disease, a leading cause of stroke and dementia.
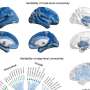
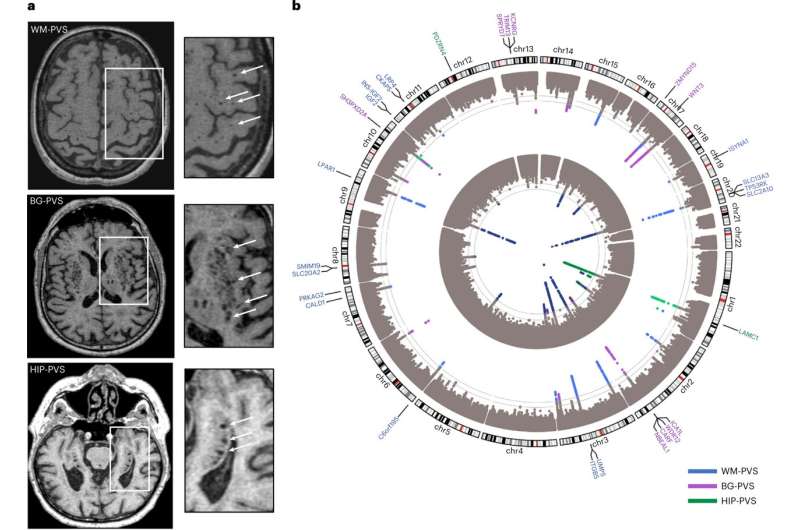
In the study, "Genomics of perivascular space burden unravels early mechanisms of cerebral small vessel disease," researchers employed a genome-wide association study of 40,095 participants with brain imaging data in 18 population-based cohorts (66.3 ± 8.6 yr, 96.9% European ancestry). The analysis revealed 24 genome-wide significant PVS risk loci, mainly in the white matter.
These loci were already associated with white matter PVS in young adults (N = 1,748; 22.1 ± 2.3 yr) and enriched in early-onset leukodystrophy (abnormal growth of white matter) genes and genes expressed in fetal brain endothelial cells, suggesting early-life mechanisms. A Research Briefing discussing the findings has been published in the same journal issue.
PVS are physiological spaces surrounding small vessel walls running from the subarachnoid space through the brain parenchyma. In experimental models, PVS appear to be important conduits for substrate delivery, flushing interstitial fluid, clearing metabolic waste like beta-amyloid peptide, and is involved in brain fluid regulation as part of the "glymphatic system."
Mounting evidence suggests a major role of PVS in cerebral injury, stroke, and dementia. Dilation of PVS observed on brain magnetic resonance imaging is thought to be a marker of dysfunction and is speculated to reflect impairment of brain fluid and waste clearance. The incidences of PVS increase with age and vascular risk factors like hypertension, and they are associated with a host of white matter and vascular-related brain diseases.
Several studies have also suggested associations with the number of visible PVS on brain MRI with stroke, Alzheimer's disease and cerebral amyloid angiopathy. Post-stroke edema has been linked to post-stroke PVS enlargement.
Researchers conducted a genome-wide association study (GWAS) meta-analyses with 40,095 participants and whole-exome/whole-genome sequencing (WES/WGS) of another 19,010. They ran analyses separately for white matter (WM)-PVS, basal ganglia (BG)-PVS and hippocampal (HIP)-PVS.
With a decent data set collected, the bioinformatics exploration delved into tissue and cell-specific gene expression databases and drug target libraries of identified PVS risk loci.
The study identified 24 independent genome-wide significant risk loci for extensive PVS burden. Two-thirds of PVS risk loci point to pathways not mediated by established risk factors and involve the extracellular matrix, the blood-brain barrier, membrane transport and vascular development.
There was significant evidence for causal associations between high blood pressure and PVS and between PVS and stroke for BG. Significant enrichment of PVS loci in genes involved in early-onset leukodystrophies and expressed in fetal brain vascular endothelial cells suggests the involvement of developmental processes and in previously investigated targets of drugs for vascular and cognitive disorders.
The researchers conclude that the study findings "...provide insight into the biology of PVS across the adult lifespan and its contribution to cerebral small vessel disease pathophysiology, with potential for genetically informed prioritization of drug targets for prevention trials of cerebral small vessel disease, a major cause of stroke and dementia worldwide."
More information: Marie-Gabrielle Duperron et al, Genomics of perivascular space burden unravels early mechanisms of cerebral small vessel disease, Nature Medicine (2023). DOI: 10.1038/s41591-023-02268-w
Genomics of perivascular space burden across the lifespan and across ancestries, Nature Medicine (2023). DOI: 10.1038/s41591-023-02269-9
© 2023 Science X Network