This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New gene implicated in cancer, cellular stress response
Northwestern Medicine scientists have identified a gene that plays a role in cellular responses to molecular stressors, such as DNA damage and nutrient scarcity, according to findings published in Nature Communications.
Marc Mendillo, Ph.D., assistant professor of Biochemistry and Molecular Genetics and senior author of the study, built upon his previous work identifying and characterizing HAPSTR1, which was found to be expressed in multiple types of cancer cells.
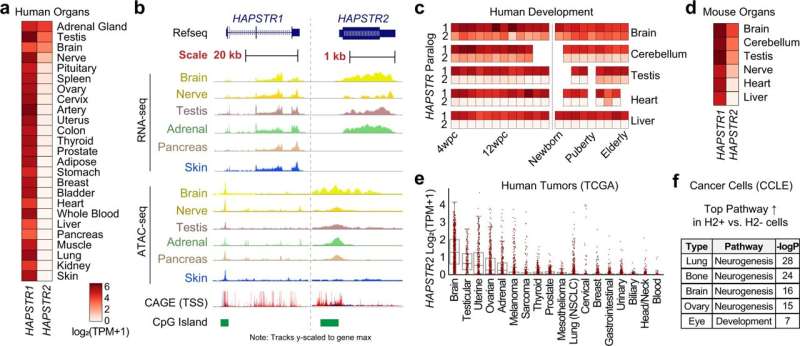
The new gene, HAPSTR2, is expressed in the brain, testis, and in certain cancers, according to the study.
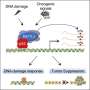
All human cells contain signaling pathways that enable them to counteract different types of cellular and environmental stressors. However, because these stress response pathways are almost always studied individually, the mechanisms which help regulate and link different stress response pathways have remained poorly understood.
In a previous study, Mendillo and his collaborators found HAPSTR1 both interacts with and is degraded by a protein called HUWE1, which has been previously implicated in different cancers and neurodevelopmental disorders. The protein is an E3 ubiquitin ligase, meaning its function is to hunt down specific other proteins and degrade them.
The previous study revealed that HUWE1 and HAPSTR1 have an unexpected relationship: HUWE1 assists HAPSTR1 in controlling stress signaling and in turn, HUWE1 feeds back to promote the degradation of HAPSTR1.
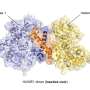
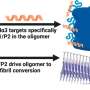
In the latest study, investigators found HAPSTR2 to be a paralog, or duplicate, of HAPSTR1.
In normal physiology, HAPSTR2 interacts with HAPSTR1 to augment protein stability independently from HUWE1. When HAPSTR1 is inhibited, HAPSTR2 takes over to buffer stress responses in cells.
The findings may help improve the understanding of how cancer and other diseases hijack stress response programming to survive, Mendillo said.
"Stress response pathways play an important protective role, but cancers co-opt these pathways to allow the cancer to survive and proliferate," said Mendillo, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. "Additionally, the HAPSTR family is present in all humans. You don't have people walking around that have obvious loss of function mutations in this gene, which leads us to believe that these genes are very important for life."
Future research will focus on understanding the mechanisms underlying HAPSTR protein function and identifying potential treatment targets for cancer.
"This HAPSTR1 protein seems to be essential for life and for cancers to develop," said David Amici, a student in the Medical Scientist Training Program and lead author of the study. "Our discovery of HAPSTR2 reveals an additional mechanism for cancer cells to ramp up this pathway. This is going to be important for therapy resistance and metastasis, things we ideally would like to turn off in cancer."
More information: David R. Amici et al, The HAPSTR2 retrogene buffers stress signaling and resilience in mammals, Nature Communications (2023). DOI: 10.1038/s41467-022-35697-1