This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers create a cell therapy based on STAb cells for a type of leukemia with few treatment options
Researchers of the Hospital Universitario 12 de Octubre in Madrid and the Josep Carreras Leukaemia Research Institute in Barcelona have developed a cell therapy for a type of leukemia which currently has very few treatment options. This STAb therapy is based on STAb-T cells and could be used for the treatment of T-Cell Acute Lymphoblastic Leukemia (T-ALL) in those patients for whom chemotherapy or bone marrow transplantation have not worked.
STAb-T therapy is an evolution of the so-called CAR-T therapies that are currently revolutionizing cancer treatment. CAR-T therapies are based on the modification of the patient's own immune cells, the T-lymphocytes, so that they are able to express artificial chimeric receptors that recognize and eliminate tumor cells.
The advantage of the STAb therapy over CAR-T therapy is that, while in the latter the T cell expresses a receptor with a monospecific antibody capable of recognizing one target on the tumor; the STAb therapy is based on the secretion of a special type of bispecific antibody that can recognizes two targets, one on the tumor cell and one on the T cell. In this way, the bispecific antibodies create a kind of artificial bridge that brings therapeutic T cells into contact with tumor cells, facilitating the elimination of the latter and keeping healthy T cells safe.
This distinction is essential in order to treat T-Cell Acute Lymphoblastic Leukemia. In the case of B-Cell Acute Lymphoblastic Leukemia (B-ALL), CAR-T cells recognize a single target and destroy both diseased and healthy B cells, although these patients can lead a normal life thanks to the regular supply of immunoglobulins—antibodies—obtained from healthy donors.
In T-ALL it is more difficult to apply CAR-T therapy, since the cells used to fight the tumor—T-lymphocytes—are the same ones that are diseased and their use can lead to a state of immunodeficiency that is incompatible with life. Moreover, there is no replacement therapy available, as is the case with B-cell leukemias.
T-Cell Acute Lymphoblastic Leukemia is a rapidly progressive type of leukemia resulting from the abnormal proliferation of T-cell lymphoblasts (immature white blood cells) in the bone marrow and blood. It is a so-called rare disease that accounts for about 10–15% of all acute leukemias diagnosed in children and 20–25% of those affecting adults. In total, approximately 100 cases are detected each year in Spain.
Therapeutic innovation at 12 de Octubre Hospital
STAb-T therapy for the treatment of T-ALL was created by the Joint Cancer Immunotherapy Clinical Research Unit of the Hospital Universitario 12 de Octubre and the Spanish National Cancer Research Center (CNIO), and led by Dr. Luis Álvarez-Vallina and the team of the Josep Carreras Leukaemia Research Institute, Dr. Pablo Menéndez and Dr. Diego Sánchez-Martínez. This therapy could be an improvement over CAR-T, especially in relapsed patients with a reduced number of normal T lymphocytes.
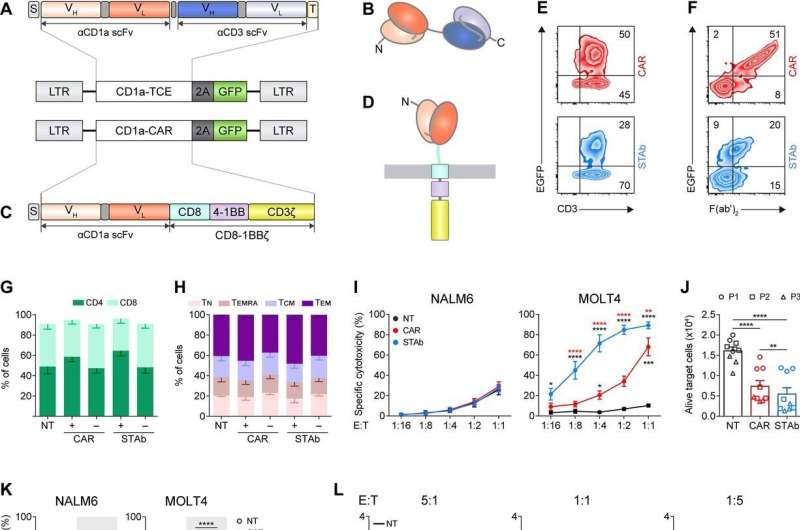
In the paper "Efficient preclinical treatment of cortical T cell acute lymphoblastic leukemia with T lymphocytes secreting anti-CD1a T cell engagers," published in Journal for ImmunoTherapy of Cancer, researchers Anaïs Jiménez-Reinoso, Néstor Tirado and other members of the team have shown that STAb-T cells work very efficiently both in vitro and in vivo animal models. Different options are currently being considered to bring this therapy to clinical trials.
Immunotherapy strategies and adoptive cell therapies still benefit few patients. "It is necessary to develop strategies addressed to very specific targets for each disease and adapted for each patient," explains Dr. Alvarez-Vallina. In his opinion, "the future in cancer and leukemia research lies in the creation of personalized therapies that provide options for all those who today find no alternative to conventional therapies. STAb-T therapy is on this path."
Dr. Alvarez-Vallina concludes, "In the case of CAR-T, many hospitals are like a production center of the therapy. Regarding STAb-T cells, this is a completely new therapy that has arisen at the Hospital Universitario 12 de Octubre and represents an innovation in the field of cell therapies." It is important to note that STAb-T therapy can be applicable to multiple types of cancer and some of these modalities are in clinical development.
More information: Anaïs Jiménez-Reinoso et al, Efficient preclinical treatment of cortical T cell acute lymphoblastic leukemia with T lymphocytes secreting anti-CD1a T cell engagers, Journal for ImmunoTherapy of Cancer (2022). DOI: 10.1136/jitc-2022-005333