This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Largest study to date of lebrikizumab supports its use for treatment of atopic dermatitis

Two phase 3 trials suggest that a 16 week treatment with a drug called lebrikizumab was effective in adults and adolescents with moderate to severe dermatitis, according to a paper published in the New England Journal of Medicine.
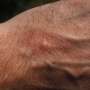
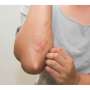
Atopic dermatitis is the most prevalent chronic inflammatory skin disease with a worldwide prevalence of 20% among children and up to 7% among adults. The condition causes inflamed skin, itching and is associated with an impaired quality of life.
"These are the largest studies of lebrikizumab in atopic dermatitis to date," Jonathan I. Silverberg, M.D., the co-investigator of the study and associate professor of Dermatology at George Washington University School of Medicine & Health Sciences, "Our results showed lebrikizumab to be safe, highly-effective and well tolerated in patients with moderate to severe atopic dermatitis."
Silverberg and his colleagues conducted two identically designed phase 3 trials to evaluate the safety and efficacy of using this drug. In the first trial, the team randomly assigned 283 patients to the active drug group and 141 patients to the placebo group. In the second trial 281 patients got lebrikizumab and 146 patients were in the placebo group.
After 16 weeks, the team found that lebrikizumab therapy led to improvements, as compared to placebo, in the outcomes of the study. For example, the drug led to significantly clearer skin, reduced itching and thus helped improve sleep.
The researchers note that 16 weeks is not enough time to assess long-term safety, however this study suggests only mild or moderate side effects with a low incidence. Additional study will need to be done to prove the long-term safety and efficacy of the drug, Silverberg said.
The findings also confirm the central role of interleukin-13 in the pathogenesis of the disease. The researchers believe that lebrikizumab binds with interleukin-13 and thus leads to improvements in dermatitis.
Silverberg will be presenting his paper at the 2023 American Academy of Dermatology (AAD) Meeting, March 17–21 in New Orleans, LA. The study, "Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Dermatitis," was published March 15 in the New England Journal of Medicine.
More information: Jonathan I. Silverberg et al, Two phase 3 trials of lebrikizumab for moderate-to-severe dermatitis, New England Journal of Medicine (2023). DOI: 10.1056/NEJMoa2206714. www.nejm.org/doi/full/10.1056/NEJMoa2206714