This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
DNA treatment could delay paralysis that strikes nearly all patients with ALS
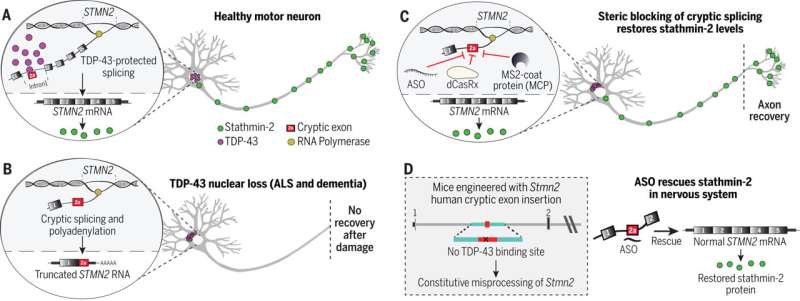
In virtually all persons with amyotrophic lateral sclerosis (ALS) and in up to half of all cases of Alzheimer's disease (AD) and frontotemporal dementia, a protein called TDP-43 is lost from its normal location in the nucleus of the cell. In turn, this triggers the loss of stathmin-2, a protein crucial to regeneration of neurons and the maintenance of their connections to muscle fibers, essential to contraction and movement.
Writing in the March 16, 2023 issue of Science, a team of scientists, led by senior study author Don Cleveland, Ph.D., Distinguished Professor of Medicine, Neurosciences and Cellular and Molecular Medicine at University of California San Diego School of Medicine, with colleagues and elsewhere, demonstrate that stathmin-2 loss can be rescued using designer DNA drugs that restore normal processing of protein-encoding RNA.
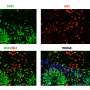
"With mouse models we engineered to misprocess their stathmin-2 encoding RNAs, like in these human diseases, we show that administration of one of these designer DNA drugs into the fluid that surrounds the brain and spinal cord restores normal stathmin-2 levels throughout the nervous system," Cleveland said.
Cleveland is broadly credited with developing the concept of designer DNA drugs, which act to either turn on or turn off genes associated with many degenerative diseases of the aging human nervous system, including ALS, AD, Huntington's disease and cancer.
Several designer DNA drugs are currently in clinical trials for multiple diseases. One such drug has been approved to treat a childhood neurodegenerative disease called spinal muscular atrophy.
The new study builds upon ongoing research by Cleveland and others regarding the role and loss of TDP-43, a protein associated with ALS, AD and other neurodegenerative disorders. In ALS, TDP-43 loss impacts the motor neurons that innervate and trigger contraction of skeletal muscles, causing them to degenerate, eventually resulting in paralysis.
"In almost all of instances of ALS, there is aggregation of TDP-43, a protein that functions in maturation of the RNA intermediates that encode many proteins. Reduced TDP-43 activity causes misassembly of the RNA-encoding stathmin-2, a protein required for maintenance of the connection of motor neurons to muscle," said Cleveland.
"Without stathmin-2, motor neurons disconnect from muscle, driving paralysis that is characteristic of ALS. What we have now found is that we can mimic TDP-43 function with a designer DNA drug, thereby restoring correct stathmin-2 RNA and protein level in the mammalian nervous system."
Specifically, the researchers edited genes in mice to contain human STMN2 gene sequences and then injected antisense oligonucleotides—small bits of DNA or RNA that can bind to specific RNA molecules, blocking their ability to make a protein or changing how their final RNAs are assembled—into cerebral spinal fluid. The injections corrected STMN2 pre-mRNA misprocessing and restored stathmin-2 protein expression fully independent of TDP-43 function.
"Our findings lay the foundation for a clinical trial to delay paralysis in ALS by maintaining stathmin-2 protein levels in patients using our designer DNA drug," Cleveland said.
More information: Michael W. Baughn et al, Mechanism of STMN2 cryptic splice-polyadenylation and its correction for TDP-43 proteinopathies, Science (2023). DOI: 10.1126/science.abq5622