New molecular clues on neurodevelopmental disorder similar to Angelman syndrome
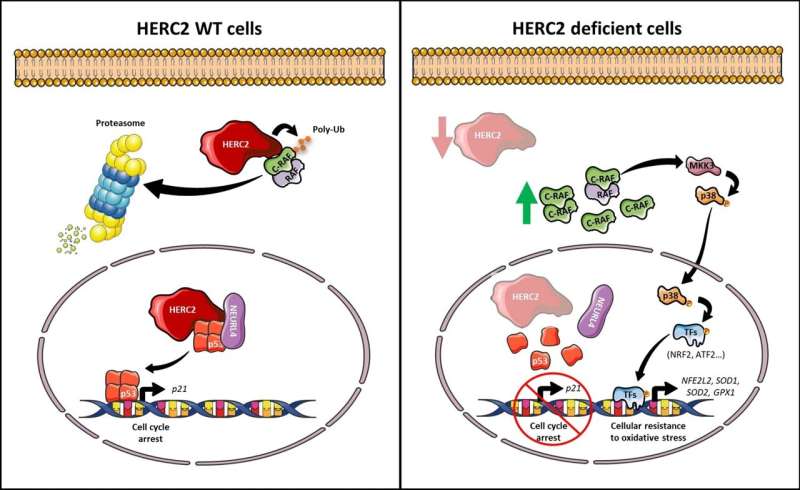
Angelman syndrome is a genetic disease that causes a developmental delay, alterations in speech and balance, intellectual disability and sometimes, seizures. To date, researchers had identified mutations in the HERC2 gene, which encodes a ubiquitin ligase enzyme that plays a key role in the nervous system.
These mutations cause a hereditary neurodevelopmental disease that is similar to Angelman syndrome. The identified cases that are similar to Angelman syndrome have shown a total loss or very low levels of HERC2.
Now, a research group led by Professor José Luís Rosa, from the Faculty of Medicine and Health Sciences of the UB and the Bellvitge Biomedical Research Institute (IDIBELL), has analyzed the cellular signaling pathways affected by the most common mutation of the HERC2 gene (c.1781C>T, p.Pro594Leu) in this pathology.
A better understanding of how the pathogenic variants affect these pathways could be decisive to help define potential therapies for this disease.
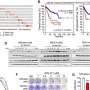
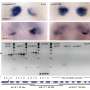
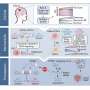
The results of the study, published in the journal Cellular and Molecular Life Sciences, show that the cells of those patients with this hereditary disease—or the human cells with a lack of HERC2 protein—have an overactivated cell response to oxidative stress, This is caused by an activation of an atypical signaling pathway in which the proteins RAF, MKK and p38 participate.
"Our results highlight the RAF inhibition as a potential therapeutic target for people with this hereditary and neurodevelopmental disorder," says Professor José Luís Rosa.
More information: Joan Sala-Gaston et al, HERC2 deficiency activates C-RAF/MKK3/p38 signalling pathway altering the cellular response to oxidative stress, Cellular and Molecular Life Sciences (2022). DOI: 10.1007/s00018-022-04586-7