This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Patients in clinical trial for prostate cancer report no decline in quality of life at one year post-treatment
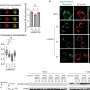
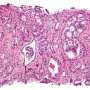
The patient-reported outcomes (PROs) of the phase 3 NRG Oncology clinical trial RTOG 0815 comparing dose-escalated radiotherapy (RT) alone to dose-escalated RT combined with short-term (6 months) androgen deprivation therapy (STAD) indicate that the addition of STAD did not significantly impact urinary or bowel quality of life but did significantly decrease hormone and sexual quality of life. However, this decline in quality of life was temporary and there were no clinically meaningful differences in quality of life between treatment arms by one year after the initiation of treatment. These results of this study, led by Benjamin Movsas, were recently published in the Journal of Clinical Oncology.
The study also recently reported results that were also published in the Journal of Clinical Oncology that indicated that the experimental treatment arm of dose-escalated RT with the STAD did not improve overall survival outcomes for men with intermediate-risk prostate cancer as initially hypothesized but, the use of STAD did improve other clinical outcomes such as rates of biochemical failure, distant metastases, and prostate cancer-specific mortality.
NRG-RTOG 0815 accrued 1,492 patients with intermediate risk prostate cancer and randomly assigned patients to receive either dose-escalated RT alone (external beam RT to 79.2 Gy, or external beam to 45 Gy with brachytherapy boost) or dose-escalated RT with 6 months of STAD with LHRH agonist/antagonist therapy plus anti-androgen. In all, 420 patients agreed to participate in the quality of life component of the trial.
Primary PRO in this analysis was measured by the Expanded Prostate Cancer Index Composite (EPIC) which is comprised of four individually validated domains: urinary, bowel, sexual, and hormonal domain scores. Secondary PROs were measured using the Patient-Reported Outcome Measurement Information System (PROMIS) Fatigue short form. The Godin Leisure-Time Exercise Questionnaire (GTLTEQ), the Pittsburgh Sleep Quality Index (PSQI), and the EQ-5D self-assessment were also used to assess PROs. Assessments were performed at the end of RT, and at months 6, 12, and 60.
The primary PRO completion rates using EPIC were excellent at greater than or equal to 86% through the first year of follow-up, then 70%–75% at 5 years. There were significant clinically meaningful (p<0.0001) deficits in the EPIC hormonal and sexual domains in Arm 2, combining dose-escalated RT with STAD at completion of RT and the 6-month follow-up. However, at one year, there were no significant clinically meaningful differences between arms and at the 5-year follow-up, the scores for both EPIC domains returned to baseline. There were also no significant clinically meaningful differences at any time points between arms for PROMIS-fatigue, EQ5D, and EPIC bowel or urinary scores.
"While there is an initial decline in the hormone and sexual quality of life for men that received short term hormones in addition to radiation, it is reassuring that this impact was temporary and that quality-of-life outcomes were not clinically meaningfully different between arms by one year," stated Benjamin Movsas, MD, Medical Director of the Henry Ford Health Cancer and the lead author of the NRG-RTOG 0815 PROs publication.
"Patient-reported outcomes such as these are incredibly valuable to help individuals make informed decisions when determining their treatment options. We are particularly pleased that the clinical outcomes paper and the patient reported outcomes paper for NRG RTOG 0815 were published in the same issue of the Journal of Clinical Oncology, as together they tell the 'whole story' for this important clinical setting," he said.
More information: Benjamin Movsas et al, Dose-Escalated Radiation Alone or in Combination With Short-Term Total Androgen Suppression for Intermediate-Risk Prostate Cancer: Patient-Reported Outcomes From NRG/Radiation Therapy Oncology Group 0815 Randomized Trial, Journal of Clinical Oncology (2023). DOI: 10.1200/JCO.22.02389
Daniel J. Krauss et al, Dose-Escalated Radiotherapy Alone or in Combination With Short-Term Androgen Deprivation for Intermediate-Risk Prostate Cancer: Results of a Phase III Multi-Institutional Trial, Journal of Clinical Oncology (2023). DOI: 10.1200/JCO.22.02390