This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New study reveals how lung cells protect themselves against RNA viral infection
A new University of California, Irvine-led study uncovers how a protein called APOBEC3B could protect cells against many different types of RNA viruses like respiratory syncytial virus (RSV), SARS-CoV2, influenza virus, poliovirus and measles, helping to prevent disease. The study was published in Nature Communications.
The study findings provide an understanding of how lung cells, in particular, protect themselves against RNA viral infection. This new discovery is essential to developing future therapies to limit viral infection and improve the health of patients with chronic lung disease.
Respiratory syncytial virus (RSV), SARS-CoV2, influenza virus, poliovirus, and measles—all single-stranded RNA viruses—are some examples of highly contagious diseases transmitted by respiratory aerosols that commonly infect lung cells.
"Patients with chronic lung diseases, such as asthma, cystic fibrosis, chronic obstructive pulmonary disease and interstitial lung diseases, are more susceptible to respiratory lung infections. These viral infections can further contribute to disease progression," said Remi Buisson, Ph.D., assistant professor in the UCI School of Medicine Department of Biological Chemistry. "An exciting part of our finding fills a critical knowledge gap by illuminating how APOBEC3B can promote innate immune responses in host cells without generating mutations in the virus genomes and promoting viral evolution."
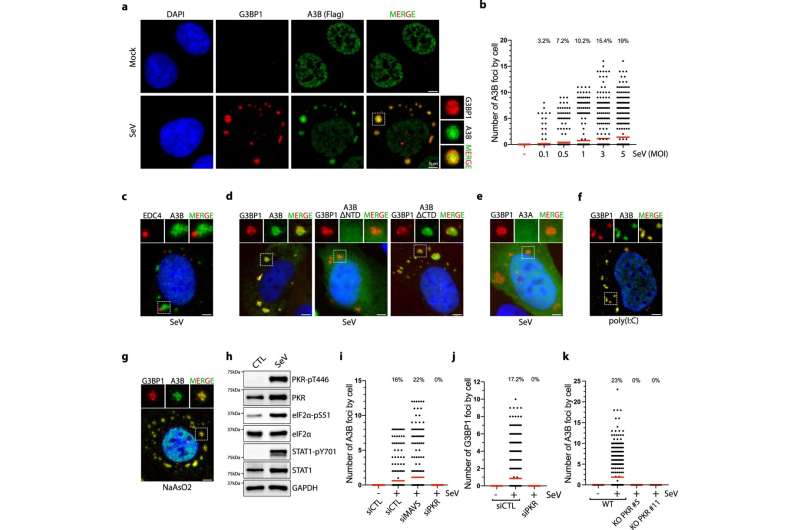
In this study, graduate students Lavanya Manjunath and Sunwoo Oh, both at UCI School of Medicine in the Buisson Laboratory, utilized different RNA virus models, including Sendai virus, poliovirus, and Sindbis virus as tools to determine how APOBEC3B regulates innate immune signaling in response to viral infection. Moreover, they found that APOBEC3B is recruited to stress granules through its interaction with PABPC1 to prevent stress granule destabilization and protect mRNAs associated with stress granules from an RNA endonuclease RNase L that cleave RNAs in host cells.
"We propose that APOBEC3B, in addition to its canonical role to edit viral genomes, functions with PABPC1 as important innate immunity mediators, protecting cells at different steps of the innate immune response against viral infections," said Buisson.
More research is needed to develop strategies to prevent RNA viral infection.
"Our next steps are to determine the detailed mechanism of how APOBEC3B recognizes viral genome to promote an innate immune response and prevent viral replication," said Buisson. "The goal is to identify how RNA viruses developed resistance mechanisms to counteract APOBEC3B functions and escape host cell defense."
More information: Lavanya Manjunath et al, APOBEC3B drives PKR-mediated translation shutdown and protects stress granules in response to viral infection, Nature Communications (2023). DOI: 10.1038/s41467-023-36445-9