2021 market access prospects for Spain
pharmaphorum
FEBRUARY 24, 2021
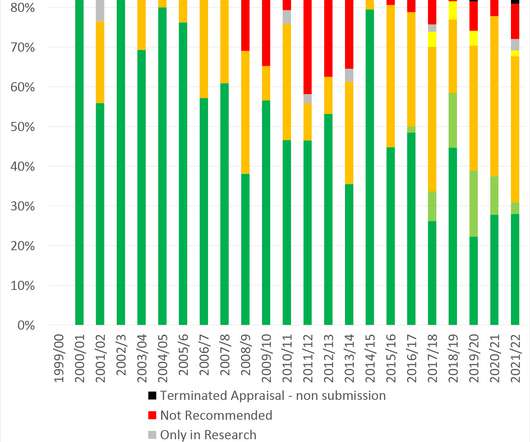
In the latest of a suite of features looking at the biggest markets in Europe, Leela Barham takes stock of what 2021 could bring for market access in Spain. About the author. Leela worked as an advisor to the Department of Health and Social Care on the 2019 Voluntary Scheme for Branded Medicines Pricing and Access (VPAS).




























Let's personalize your content