Epigenetic Editing Explodes on the Heels of Gene Editing Success
BioSpace
MARCH 24, 2024
Ubiquitous potential, possible safety advantages and the recent growth of cell and gene therapy are driving investment in a different type of genetic editing.
BioSpace
MARCH 24, 2024
Ubiquitous potential, possible safety advantages and the recent growth of cell and gene therapy are driving investment in a different type of genetic editing.

WCG Clinical
MARCH 25, 2024
This content is password protected. To view it please enter your password below: Password: The post How Latinas are Shaping Clinical Research appeared first on WCG.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Rethinking Clinical Trials
MARCH 28, 2024
Dr. Rosa Gonzalez-Guarda The NIH Pragmatic Trials Collaboratory’s Health Equity Core developed a written aid to offer guidance on inclusive language and terms to use when referring to specific people, groups, and communities. The Equitable Language Cheat Sheet is available on the Health Equity Core webpage and will be updated as terminology and guidance evolves.

Velocity Clinical Research
MARCH 26, 2024
Last month, Nick Spittal , Velocity’s chief operating officer announced the company’s latest partnership with HealthMatch and results from an unprecedented patient recruitment pilot at the SCOPE conference in Orland, Florida. He was joined by HealthMatch CEO, Manuri Gunawardena, on stage for a joint presentation, as Clinical Trials Report journalist Michael Causey reports: Velocity announced its partnership with HealthMatch on an innovative patient-centric recruitment pilot during the session, “

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Outsourcing Pharma
MARCH 26, 2024
Today (March 26) One2Treat, a new innovative start-up offering progressive digital health solutions, has launched a pioneering approach addressing two of the most strategic challenges in the biopharma industry today.

Bio Pharma Dive
MARCH 25, 2024
Leveraging real-world data (RWD) transforms global clinical trials, enhancing patient engagement and improving regulatory decision-making.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
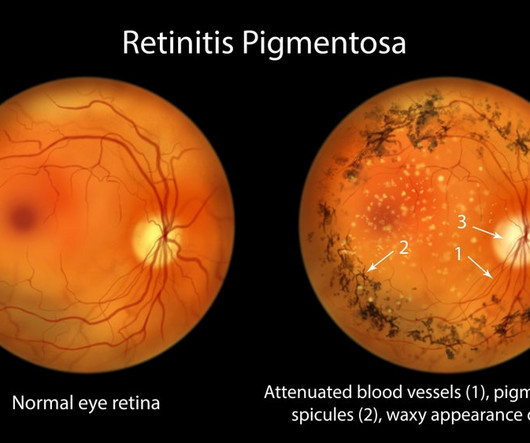
Pharmaceutical Technology
MARCH 28, 2024
Nanoscope Therapeutics released promising top-line results for its RESTORE trial, which is studying the gene therapy MCO-010 in patients with retinitis pigmentosa (RP).

Rethinking Clinical Trials
MARCH 27, 2024
Drs. Amit Garg and Stephanie Dixon In this Friday’s PCT Grand Rounds, Amit Garg and Stephanie Dixon of Western University’s Schulich School of Medicine and Dentistry will present “Effect of a Multicomponent Intervention to Improve Patient Access to Kidney Transplant and Living Kidney Donation: A Pragmatic, Cluster-Randomized Trial.” The Grand Rounds session will be held on Friday, March 27, 2024, at 1:00 pm eastern.

Bio Pharma Dive
MARCH 28, 2024
Xilio will get just over $40 million from Gilead in return for a license to its experimental IL-12 immunotherapy. The biotech is also discontinuing other work and laying off staff.

BioSpace
MARCH 25, 2024
The National Institutes of Health claims BioNTech is in default regarding alleged royalty payments the agency contends it is owed in connection with the company’s COVID-19 vaccine Comirnaty.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Pharmaceutical Technology
MARCH 28, 2024
According to the CDC, over 64 cases of measles have been documented in the US in 2024, already exceeding 2023's total of 58.

Fierce Pharma
MARCH 25, 2024
Even as Germany's BioNTech deals with ongoing declines of its revenue and share price, it's facing another serious concern: U.S. | Even as Germany's BioNTech deals with ongoing declines of its revenue and share price, it's facing another serious concern: U.S. officials are pressing the company to pay royalties linked to the commercialization of its lucrative Pfizer-partnered COVID-19 vaccine.

Bio Pharma Dive
MARCH 25, 2024
The agency wants Regeneron to make more progress with a confirmatory trial before clearing odronextamab, a “bispecific” antibody being developed for multiple blood cancers.

pharmaphorum
MARCH 26, 2024
Nanoscope Therapeutics is on the brink of filing for FDA approval of what could be the first gene therapy for incurable eye disease retinitis pigmentosa (RP) that can be used regardless of underlying genetic mutations.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharmaceutical Technology
MARCH 25, 2024
The US FDA approved Johnson & Johnson's (J&J) OPSYNVI for chronic treatment in adult patients with pulmonary arterial hypertension.

Fierce Pharma
MARCH 25, 2024
In 2023, Eli Lilly CEO David Ricks received a 24% boost in pay from $21.4 million to $26.6 million. The increase coincides with the company's booming sales.

Bio Pharma Dive
MARCH 29, 2024
Confirmatory results for Krazati, which Bristol Myers acquired via its buyout of Mirati, could help the drug win full approval while Amgen has been set back.

BioSpace
MARCH 26, 2024
While Pfizer has ended one of its two Phase III studies for inclacumab in sickle cell disease, the company is still eyeing an approval for the antibody in the inherited blood disorder by 2026.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
Pharmaceutical Technology
MARCH 26, 2024
The US FDA has approved Alexion’s Ultomiris to treat adults with AQP4 Ab+ neuromyelitis optica spectrum disorder (NMOSD).
Fierce Pharma
MARCH 26, 2024
After three years, the crown jewel of Merck’s $11.5 billion acqu | After three years, the crown jewel of Merck’s $11.5 billion acquisition of Acceleron is ready to pay dividends. With the FDA’s approval of Winrevair (sotatercept) to treat pulmonary arterial hypertension (PAH), Merck is set to launch the first disease-modifying treatment for the condition.

Bio Pharma Dive
MARCH 26, 2024
Merck is couting on Winrevair, which it acquired by buying Acceleron Pharma, to help soften the blow when Keytruda loses patent protection later this decade.

BioSpace
MARCH 24, 2024
The regulator has allowed for emergency use of Invivyd’s monoclonal antibody Pemgarda as a COVID-19 pre-exposure prophylaxis for moderately or severely immunocompromised patients.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharmaceutical Technology
MARCH 25, 2024
Cancer Research UK and the CRIS Cancer Foundation have awarded a £1.7m ($2.1m) grant for developing the lung cancer vaccine LungVax.
Fierce Pharma
MARCH 25, 2024
With the FDA expected this week to approve a first-in-class treatment from Merck for pulmonary arterial hypertension (PAH), Johnson & Johnson has beaten its New Jersey rival to the punch with a | With the FDA expected this week to approve a first-in-class treatment from Merck for pulmonary arterial hypertension (PAH), Johnson & Johnson has beaten its New Jersey rival to the punch with a nod from the U.S. regulator for its newest PAH treatment.

Bio Pharma Dive
MARCH 26, 2024
The results are from a small Phase 1 study, but suggest Viking’s oral GLP-1 drug may not come with high rates of gastrointestinal side effects.

BioSpace
MARCH 26, 2024
Moderna has entered into a development and commercialization funding agreement with asset management firm Blackstone Life Sciences to help advance its pipeline of flu vaccine candidates.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Technology
MARCH 26, 2024
The approval from Japan’s Ministry of Health, Labour, and Welfare (MHLW) is based on positive results from two studies.
pharmaphorum
MARCH 27, 2024
Brainomix's e-Lung AI can accurately identify idiopathic pulmonary fibrosis patients most likely to progress and could inform the clinical trial design for new therapies

Bio Pharma Dive
MARCH 28, 2024
The agency gave Akebia’s drug an OK after receiving more safety data, but imposed a strict boxed warning for its use that may limit uptake.
BioSpace
MARCH 24, 2024
Drugmakers are testing a variety of biologics and small molecules in conjunction with therapies aimed at modulating the immune system to target and destroy cancer cells.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Let's personalize your content