Generative AI-focused biotech startup Evozyne raises $81m
Pharmaceutical Technology
SEPTEMBER 28, 2023
The company’s algorithms put proteins through millions of years of simulated evolution to identify potential functional candidates.

Pharmaceutical Technology
SEPTEMBER 28, 2023
The company’s algorithms put proteins through millions of years of simulated evolution to identify potential functional candidates.

Bio Pharma Dive
SEPTEMBER 28, 2023
In a note to clients, Evercore ISI analyst Jonathan Miller described the Project NextGen contract for Gritstone as “certainly a nice signal of continued government support for COVID research.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Rethinking Clinical Trials
SEPTEMBER 28, 2023
Speaker Tumaini Rucker Coker, MD, MBA Professor of Pediatrics Division Head for General Pediatrics University of Washington Department of Pediatrics Seattle Children’s Hospital Slides Keywords Pediatrics, Preventive Medicine, Community Health, Well Child Care Key Points There are 10 preventive care visits from ages 0-3, usually scheduled as 15-20 minute visits with a pediatrician.

Bio Pharma Dive
SEPTEMBER 28, 2023
Interim results from a study called “Mariposa” found that a regimen of two J&J medicines improved progression-free survival versus AstraZeneca’s widely used therapy.
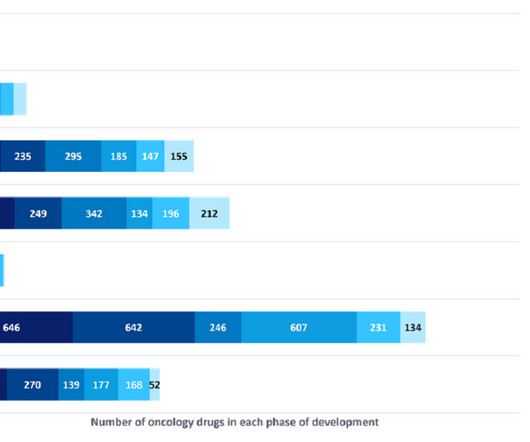
Pharmaceutical Technology
SEPTEMBER 28, 2023
It is common for biopharmaceutical companies that start as private entities to become public companies soon after their key pipeline assets reach the clinical stage of development, in order to unlock the large amount of capital needed to run costly clinical trials.

Bio Pharma Dive
SEPTEMBER 28, 2023
The drug’s success in two late-stage clinical trials has buoyed Karuna to a market valuation exceeding $6 billion.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
SEPTEMBER 28, 2023
The test developer will use the Series B funding to complete a 10,000-person clinical trial ahead of a planned launch in 2025.

Pharmaceutical Technology
SEPTEMBER 28, 2023
Pharmaceutical Technology has listed some of the leading providers of product inspection, testing and detection equipment and services in the pharmaceutical industry.
BioSpace
SEPTEMBER 28, 2023
Pivotal clinical trials in Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis and multiple sclerosis are expected to read out this fall. Here's a closer look.
Pharmaceutical Technology
SEPTEMBER 28, 2023
Takeda has received approval from the US FDA for subcutaneous administration of Entyvio for ulcerative colitis in adults.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Fierce Pharma
SEPTEMBER 28, 2023
Fabre-Kramer Pharmaceuticals' major depressive disorder (MDD) drug gepirone has been rejected by the FDA not once, not twice, but three times since the turn of the millennium. | Fabre-Kramer's Exxua suffered three prior FDA rejections before scoring an FDA approval for major depressive disorder last week. Its label doesn't include sexual dysfunction as an adverse reaction, which is rare among antidepressants.

Pharmaceutical Technology
SEPTEMBER 28, 2023
Bosutinib has been approved for adult use for 10 years, but the FDA has given the green light for use in children.

Pharma Times
SEPTEMBER 28, 2023
Collaboration will enable teams across the organisation to increase clinical research innovation - News - PharmaTimes

Pharmaceutical Technology
SEPTEMBER 28, 2023
Roche signed an agreement attaining global rights to Ionis Pharmaceuticals’ programmes for Alzheimer’s disease and Huntington’s disease.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

BioSpace
SEPTEMBER 28, 2023
An FDA advisory committee this week voted overwhelmingly against BrainStorm Cell Therapeutics’ amyotrophic lateral sclerosis treatment. However, other potential therapies offer hope for ALS patients.
Pharmaceutical Technology
SEPTEMBER 28, 2023
Ono Pharmaceutical and Adimab have signed a drug discovery collaboration agreement for the development of antibody drugs in oncology
Outsourcing Pharma
SEPTEMBER 28, 2023
A study to evaluate the effect of seladelparm, a small molecule treatment by CymaBay Therapeutics, Inc. on patients with cirrhosis was announced this month (September 21).

Pharmaceutical Technology
SEPTEMBER 28, 2023
Tetra Therapeutics has received rare paediatric disease designation from the US FDA for zatolmilast to treat Fragile X syndrome (FXS).

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharma Times
SEPTEMBER 28, 2023
Initiative has been designed to support career advancement for women across the life sciences - News - PharmaTimes

Fierce Pharma
SEPTEMBER 28, 2023
Slated for an FDA decision last October, Amicus Therapeutics’ Pompe disease bid was foiled by COVID-related travel restrictions. | Slated for an FDA decision last October, Amicus Therapeutics’ Pompe disease bid was foiled by COVID-related travel restrictions. Nearly a year later, the Philadelphia company has gained its long-awaited green light.

BioSpace
SEPTEMBER 28, 2023
Thursday’s approval comes after the FDA pushed back the target action dates for Amicus’ Biologics License Application in May, allowing the regulator more time to review the company’s submitted data.

Fierce Pharma
SEPTEMBER 28, 2023
With much riding on its mRNA patent litigation against BioNTech, Germany’s CureVac thinks the case is moving in its favor. | A court in Germany suspended infringement proceedings on four patents at issue in the lawsuit filed by CureVac against BioNTech. Still, CureVac said there's reason to be optimistic its arguments may win out.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
BioSpace
SEPTEMBER 28, 2023
Following disappointing results in a mid-stage social anxiety disorder study, the Australian biotech’s investigational ion channel modulator demonstrated promising effects in patients with post-traumatic stress disorder.
Fierce Pharma
SEPTEMBER 28, 2023
While GLP-1 drugs from Novo Nordisk and Eli Lilly are believed to be relatively free of serious side effects, a few problems have emerged as the treatments have gained wider and longer-term use.
BioSpace
SEPTEMBER 28, 2023
With Intercept’s failure to emerge as the nonalcoholic steatohepatitis market leader, Madrigal Pharmaceuticals is seeking to take leadership of the space fueled by a half-a-billion-dollar public offering.

BioPharma Reporter
SEPTEMBER 28, 2023
Across the global life sciences supply chain, data, digitalization, automation, and artificial intelligence are driving the most innovation, according to a new survey from CRB.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

BioSpace
SEPTEMBER 28, 2023
After several rejections over 20-plus years, Fabre-Kramer Pharmaceuticals has secured the FDA’s approval for its major depressive disorder drug gepirone hydrochloride, now marketed under the brand name Exxua.

BioPharma Reporter
SEPTEMBER 28, 2023
Scientists from the Universities of Bath and Bristol have identified a molecule that can prevent tangling of a brain protein that is linked to diseases such as Parkinsonâs.

Fierce Pharma
SEPTEMBER 28, 2023
A highly anticipated head-to-head matchup between a Johnson & Johnson combination and AstraZeneca’s star Tagrisso as a first-line treatment in a subset of non-small cell lung cancer (NSCLC) has | With the trial win, Johnson & Johnson sees potential for its drug combination as the new standard of care in locally advanced or metastatic epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer.

BioSpace
SEPTEMBER 28, 2023
The biopharma is projecting its HIV business will reach up to $8.5 billion in sales by 2026, based on the success of its long-acting antiretroviral therapy Cabenuva.
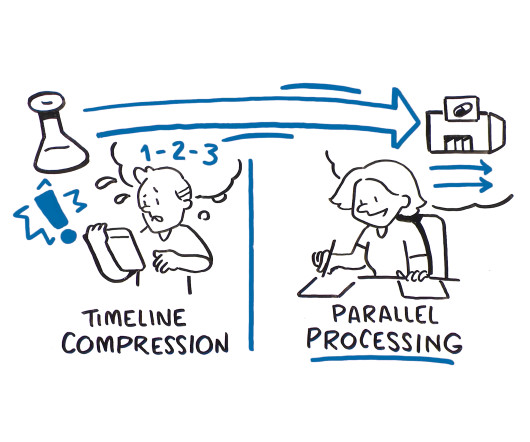
Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content