A Versant-backed biotech emerges to take on ‘overlooked’ cancer targets
Bio Pharma Dive
OCTOBER 6, 2022
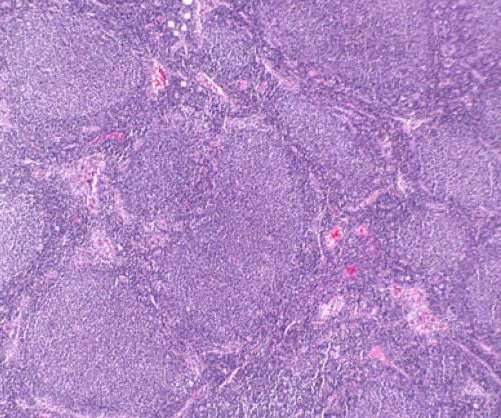
Nested Therapeutics touts a deep bench of scientific leaders and advisers, including Kevan Shokat, whose work drugging KRAS — a cancer-related gene once thought to be undruggable — helped lead to the development of Amgen’s Lumakras.































Let's personalize your content