Moderna founder unveils new drug company focused on a different kind of RNA
Bio Pharma Dive
NOVEMBER 9, 2021
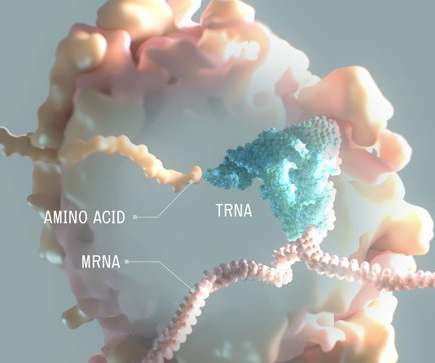
Encouraged by Moderna's success, Flagship Pioneering has been busy creating startups like Alltrna, which launched Tuesday. The biotech is researching how to make medicines from transfer RNA molecules.































Let's personalize your content