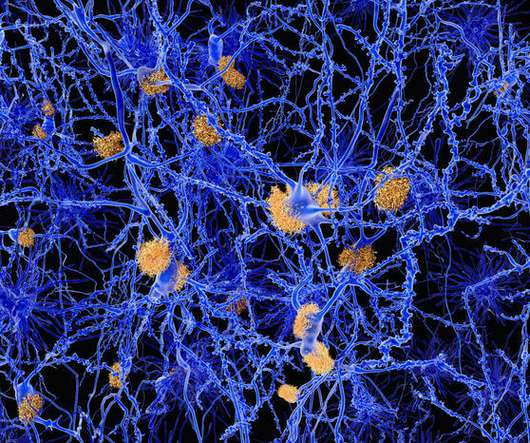
Donanemab data that demonstrated relationship between reduction of amyloid plaque and slowing of cognitive decline
The Pharma Data
JULY 29, 2021
P-tau217 in blood showed promise as additional biomarker of efficacy- Donanemab treatment led to 24% lowering of P-tau217 from baseline. In June 2021, Lilly announced the U.S. . vice president of pain and neurodegeneration, Lilly.


















Let's personalize your content