January 22, 2024: Your Pragmatic Trial Has Ended. Now What?
Rethinking Clinical Trials
JANUARY 22, 2024
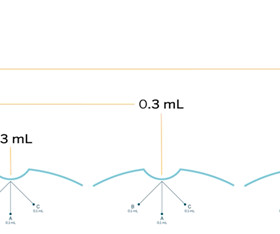
What happens to a pragmatic trial intervention after the study ends? Effective interventions may not be sustained if they require significant resources. The case studies in the article are from the ABATE Infection, LIRE, PPACT, PROVEN, STOP-CRC, and TSOS trials. Read the full article.












































Let's personalize your content