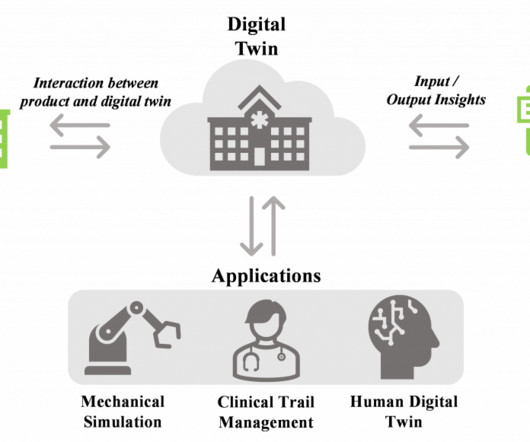
“Heigh-ho” Taiho! The PTO Says LYTGOBI Patent is Ineligible for PTE Because of Untimely Application. And a Corrected NDA Approval Letter is No Saving Grace
FDA Law Blog
APRIL 21, 2024
Government—was that, despite having missed the statutory 60-day filing deadline, the patent was granted a PTE due to a change in the law created by Section 37 of the Leahy-Smith America Invents Act (“AIA”) ( Pub. Rather, “permission for commercial marketing” was only effective upon FDA’s issuance of its corrected October 5, 2022 letter.













































Let's personalize your content