Overcoming EDC Implementation Challenges: Best Practices for Biotech Sponsors
Cloudbyz
APRIL 5, 2024
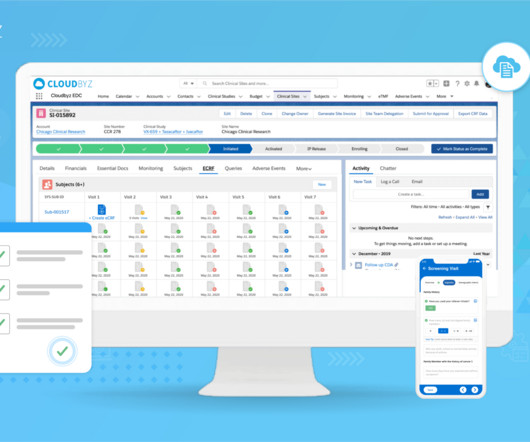
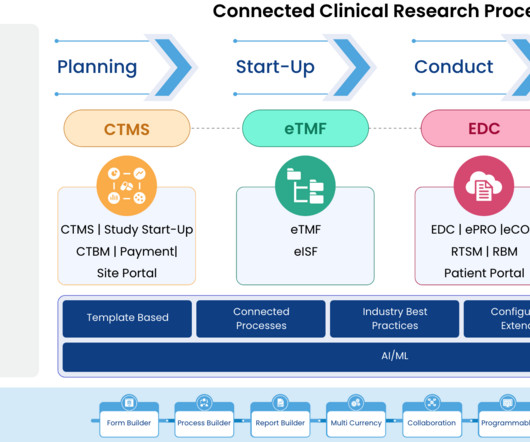
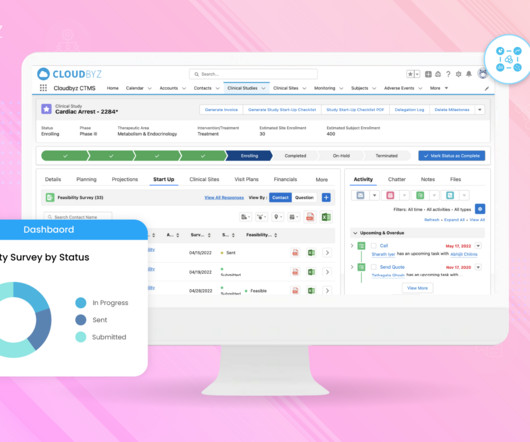
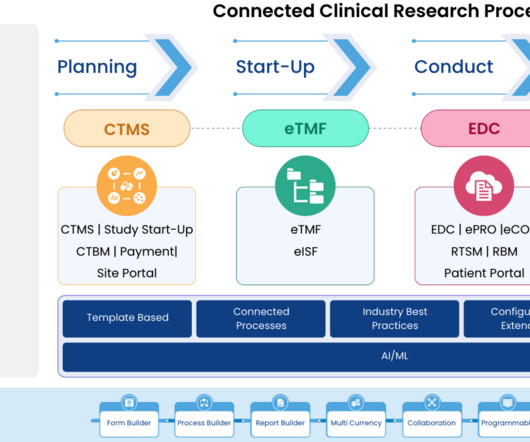
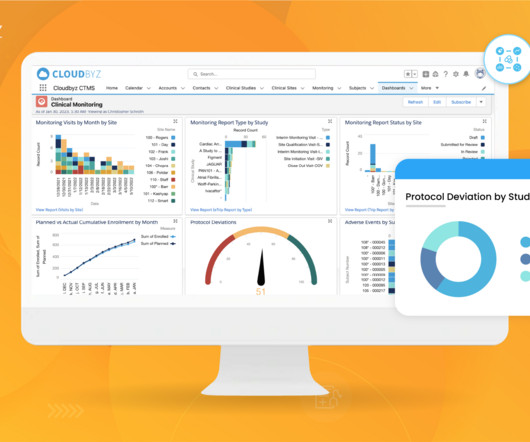
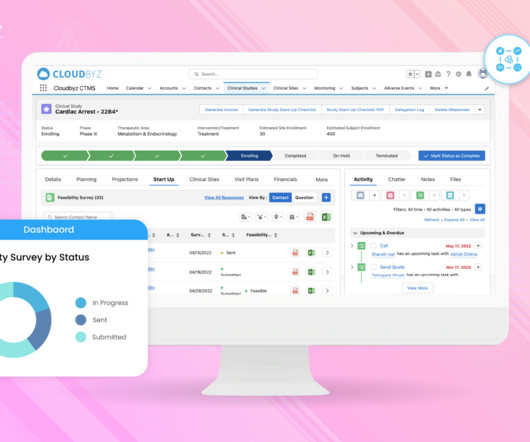
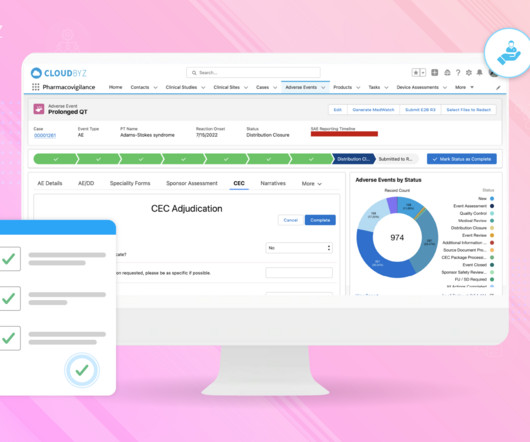
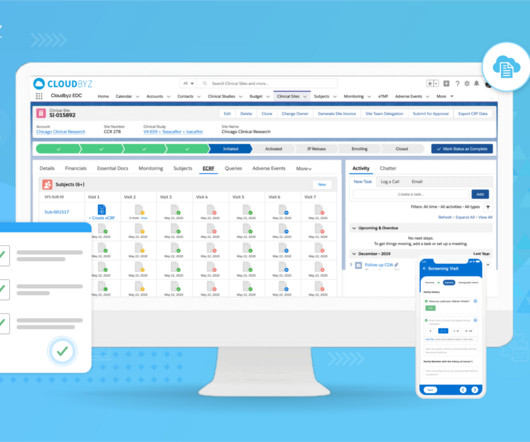
Implementing Electronic Data Capture (EDC) systems in clinical trials offers numerous benefits, including improved data accuracy, faster data availability, and streamlined processes. Best Practice: Prioritize EDC solutions that offer flexible integration capabilities with existing systems.











































Let's personalize your content