Rare Disease Day: Emmes Endpoints Solutions submits Duchenne video assessment qualification plan to FDA
Outsourcing Pharma
FEBRUARY 28, 2024
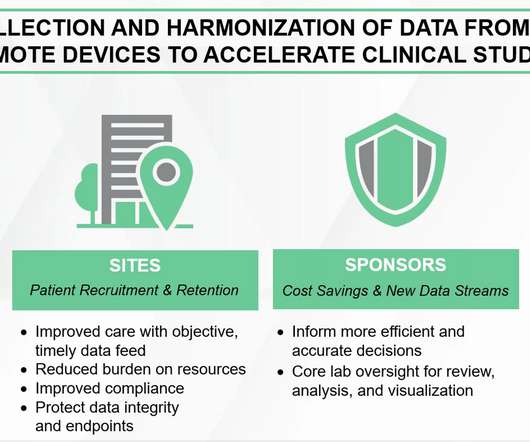
In a seminal move coinciding with Rare Disease Day, Emmes Endpoints Solutions has taken a significant step forward in the realm of rare disease research.










































Let's personalize your content