Lambda and Medidata team up to automate clinical trial data
Pharmaceutical Technology
APRIL 20, 2023
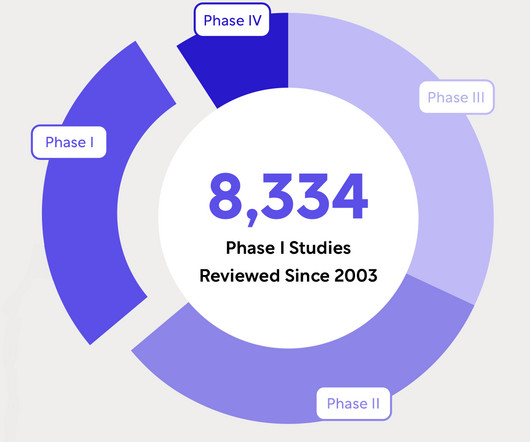
Lambda Therapeutics will employ Medidata’s Rave EDC, Rave RTSM, and Rave Imaging cloud-driven clinical solutions to access centralised data to address the entire clinical research process. After uploading, the system automates the distribution and review procedures.















































Let's personalize your content