First ever CRISPR gene therapy approval: What happens next?
BioSpace
NOVEMBER 21, 2023
CRISPR gene-editing has had its first ever approval in the UK. What can patients expect the price tag to be? Will the FDA follow suit?

 tag crispr
tag crispr 
BioSpace
NOVEMBER 21, 2023
CRISPR gene-editing has had its first ever approval in the UK. What can patients expect the price tag to be? Will the FDA follow suit?

BioTech 365
DECEMBER 1, 2020
Biotechnology, Pharma and Biopharma News – Research – Science – Lifescience ://Biotech-Biopharma-Pharma: CRISPR tagging improves accuracy of model cells grown from stem cells.A team of biomedical engineers at Duke University has created a new way to turn stem cells … Continue reading →
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
NOVEMBER 7, 2023
Vertex and CRISPR Therapeutics are up first with a Dec. | But on Tuesday bluebird said that Vertex’s price tag will not factor into how the Massachusetts company will price its treatment. Two long-awaited treatments for sickle cell disease (SCD) are on the docket for FDA decisions next month.
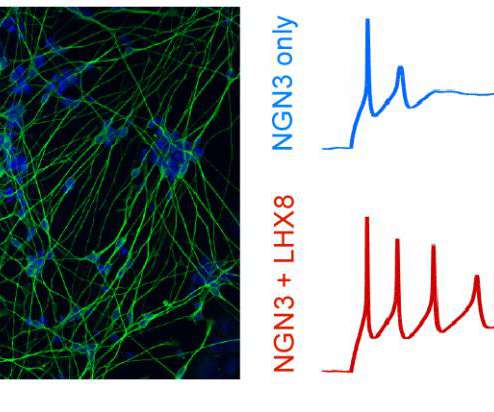
Scienmag
DECEMBER 1, 2020
Tagging produces detailed catalog of transcription factors key to making each cell type Credit: Gersbach Lab, Duke University DURHAM, N.C. – A team of biomedical engineers at Duke University has created a new way to turn stem cells into a desired cell type by mastering the language of gene regulatory networks.

Pharmaceutical Technology
APRIL 13, 2023
Released on April 12, the report focuses on bluebird bio’s lovotibeglogene autotemcel and Vertex Pharmaceuticals and CRISPR Therapeutics’ exagamglogene autotemcel or exa-cel and their potential use in treating sickle cell disease. If approved, exa-cel would be the first FDA-approved gene therapy based on CRISPR editing.

XTalks
DECEMBER 13, 2023
The landmark approvals were awarded to bluebird bio’s Lyfgenia (lovo-cel) and Vertex Pharmaceuticals and CRISPR Therapeutics’ jointly developed Casgevy (exa-cel). Casgevy is also the first ever CRISPR/Cas9-based therapy approved in the US. Vertex-CRISPR’s Casgevy has a US list price of $2.2

Pharmaceutical Technology
APRIL 5, 2023
But access to these treatments continues to remain limited due to high price tags and variable availability across regions. This includes the first potential approval of a CRISPR-based gene therapy called exa-cel , which is developed by CRISPR Therapeutics and Vertex Pharmaceuticals. There has been extremely rapid progress.
Let's personalize your content