Cannabinoids receptors: popular preclinical target but banned in 137 countries
Pharmaceutical Technology
DECEMBER 5, 2022
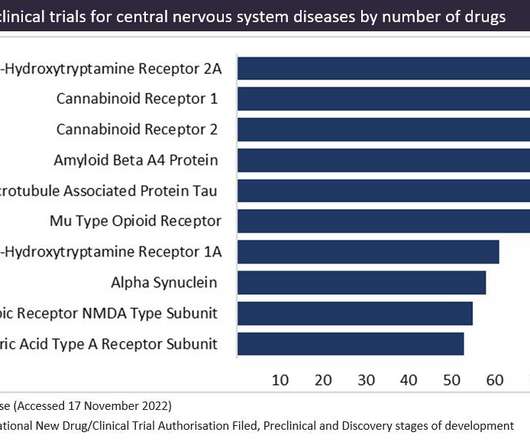
Cannabinoid receptors are a popular therapeutic target for cannabinoid-based drugs in the treatment of pain, neurological disorders and inflammation, according to GlobalData’s Pharma Intelligence Centre Drugs database. This is closely followed by CB2 receptors in second place.
















Let's personalize your content