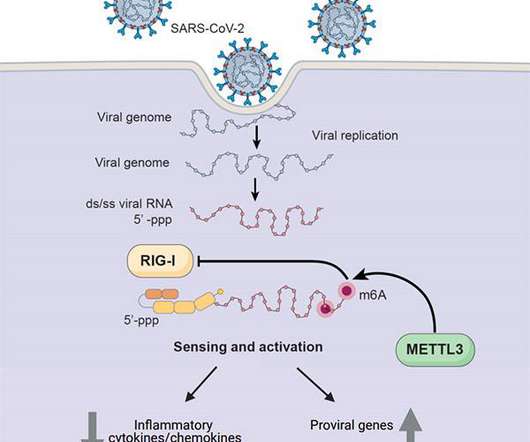
How SARS-CoV-2 hijacks human cells to evade immune system
Scienmag
APRIL 29, 2021
Credit: UC San Diego Health Sciences Researchers at University of California San Diego School of Medicine have discovered one way in which SARS-CoV-2, the coronavirus that causes COVID-19, hijacks human cell machinery to blunt the immune response, allowing it to establish infection, replicate and cause disease.
























Let's personalize your content