Genprex’s Reqorsa gene therapy picks up orphan drug designation from FDA for SCLC
Pharmaceutical Technology
AUGUST 11, 2023
The latest tag adds to three fast track designations for Reqorsa, with the company initiating a Phase I/II trial in Q4 2023.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 tag trial-design
tag trial-design 
Pharmaceutical Technology
AUGUST 11, 2023
The latest tag adds to three fast track designations for Reqorsa, with the company initiating a Phase I/II trial in Q4 2023.

Rethinking Clinical Trials
DECEMBER 6, 2023
Hughes, PhD Professor Emeritus of Biostatistics, University of Washington Slides Keywords Design, Analysis, Stepped-Wedge Trial Key Points Stepped-wedge design is typically run by clusters that are randomized. Another issue to think about with stepped-wedge trials is potential sources of variation.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JUNE 29, 2022
The US Food and Drug Administration (FDA) put a high-profile bluebird bio trial for sickle cell disease on partial clinical hold, and advisory panels deliberated over decisions involving gene therapies for amyotrophic lateral sclerosis (ALS), cerebral adrenoleukodystrophy (CALD), and beta-thalassemia.

Rethinking Clinical Trials
DECEMBER 14, 2022
Ethics, Stepped Wedge Cluster Randomized Trial, Study Design. There are two main types of clinical trials: patient randomized trial and cluster randomized trial (CRT). Trialists should work with a biostatistician to come up with the most scientifically robust design given the practical constraints of the study.

Rethinking Clinical Trials
OCTOBER 11, 2023
Moyer, PhD Statistician, NIH Office of Disease Prevention Slides Keywords Implementation; Study design; Hybrid; Clustered; DECIPHeR Key Points People often contest that hybrid designs are not as rigorous as they should be. One of the key features was that implementation measures were to be used as primary outcomes.

Rethinking Clinical Trials
AUGUST 28, 2023
Food and Drug Administration (FDA) Slides Keywords Pragmatic trials; Research; Guidance, Regulatory, Data Governance Key Points The clinical trial enterprise needs modernization. Time, cost and failure to recruit trial participants are significant barriers that must be addressed.

Rethinking Clinical Trials
AUGUST 28, 2023
Food and Drug Administration (FDA) Slides Keywords Pragmatic trials; Research; Guidance, Regulatory, Data Governance Key Points The clinical trial enterprise needs modernization. Time, cost and failure to recruit trial participants are significant barriers that must be addressed.

Rethinking Clinical Trials
JULY 9, 2023
Clinical research can exacerbate disparities, because clinical trials typically are based in urban, academic medical centers, underrepresent diverse populations, and overlook community engagement strategies in trial planning and design. Implementation mapping at the beginning of the design process is key.

Pharmaceutical Technology
JANUARY 17, 2023
On January 12, Versanis Bio announced that enrollment had begun for its Phase IIb trial, BELIEVE (NCT05616013), which aims to study bimagrumab’s (BYM-338) efficacy and safety in the US, among other locations. In addition to facilitating loss of fat, the Phase II trial also showed that a lean mass gain of 4.5% was observed in the study.

Rethinking Clinical Trials
MAY 19, 2023
Colwill Professor and Vice Chair Department of Family and Community Medicine University of Missouri Slides Keywords Electronic Health Record, Pragmatic Clinical Trial Key Points Patients bring patient-generated home blood pressure data into the clinical workflow. Koopman, MD, MS) appeared first on Rethinking Clinical Trials.

Rethinking Clinical Trials
SEPTEMBER 28, 2023
The Parent-Focused Redesign for Encounters, Newborns to Toddlers (PARENT) study was a randomized controlled trial of PARENT verses usual care for parents with infants 12 months and younger over a 12-month study period. For the first small trial, we randomized at an individual level, which takes a lot of manpower to maintain.

Rethinking Clinical Trials
FEBRUARY 13, 2023
The NITRIC Trial was a double blind, multicenter, randomized, parallel-group trial recruiting at 6 pediatric cardiac surgical centers in Australia, New Zealand, and The Netherlands. The design of platform trials can build on the lessons learned from this study. COVID-19 absorbed everyone in the middle of the trial.

Rethinking Clinical Trials
FEBRUARY 20, 2024
Speaker Jeffrey Carson, MD, MACP Principal Investigator and Study Chair MINT Trial Provost-New Brunswick, Rutgers Biomedical Health Sciences Distinguished Professor of Medicine Richard C. Trials in other clinical settings suggest the use of restrictive transfusion strategy, which is a lower hemoglobin level, is safe.

Rethinking Clinical Trials
FEBRUARY 14, 2024
It had a pragmatic recruitment model with an embedded design, multicenter study without local study investigators and management teams, and broad recruitment that included patients from all 50 states and Puerto Rico. Both drugs have a well-established safety profile and would be a good fit for a pragmatic design.
Pharmaceutical Technology
DECEMBER 5, 2022
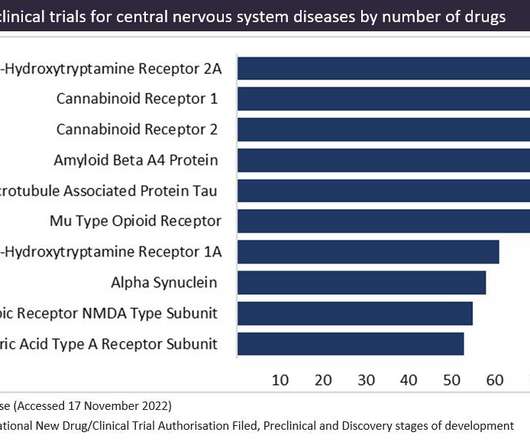
The two types of cannabinoids targeting cannabinoid receptors are endocannabinoids, which are naturally occurring endogenous ligands and synthetic cannabinoids, which are designed in a laboratory. Figure 1 shows the targets in preclinical trials for central nervous system diseases, as sourced from GlobalData’s Drugs Database.

Rethinking Clinical Trials
FEBRUARY 28, 2024
The global decentralized clinical trial market is expected to grow at a compound annual growth rate of 30.1% There is agreement that trials need to meet the people, at home and covering clinical trial deserts. Regardless of the trial inclusion and exclusion is routine. from 2021 to 2026. I think we must.
pharmaphorum
DECEMBER 24, 2021
Biogen and Eisai head towards the end of the year with some much-needed good news in their Alzheimer’s programmes, as the FDA awards a fast-track designation to lecanemab, their follow-up to recently approved Aduhelm.

Rethinking Clinical Trials
JANUARY 26, 2023
EVOLVE-MI is a pragmatic, effectiveness outcome trial of Evolocumab dosed within 10 days of a myocardial infarction (MI). EVOLVE-MI has trial innovation through its Academic Research Organizations (AROs), who are also enrolling and understand challenges firsthand. EVOLVE-MI is enrolling 4,000 patients in 3 countries.

Rethinking Clinical Trials
MAY 25, 2023
A pilot study, called the ADAPT trial, demonstrated feasibility, similar in-patient compliance and similar post-discharge adherence for the 2 options. The pragmatic randomized control trial was designed from a hospital policy perspective. Prophylaxis (chemical or mechanical) reduces risk deep vein thrombosis (DVT) by around 50%.

Rethinking Clinical Trials
OCTOBER 13, 2022
HandiCAP Trial, Pragmatic Clinical Trial. The HandiCAP trial asked the question do intraoperative handovers of anesthesia care have an impact on patient outcomes? The trial included adults 18 and older, ASA physical status III or IV, who had a major inpatient surgery with an anticipated duration of 2 hours or longer.

Rethinking Clinical Trials
OCTOBER 2, 2023
The PROTEUS Consortium’s objective is to ensure that patients, clinicians, and other decision-makers have high-quality PRO data from clinical trials and clinical practice to make the best decisions they can about treatment options. It collates and synthesizes foundational resources to create a unified, comprehensive resource.

Rethinking Clinical Trials
JANUARY 17, 2024
UPMC did not allow patients to receive experimental COVID-19 therapies outside of the context of a clinical trial and used the REMAP-CAP platform, a global pragmatic adaptive trial platform, in all clinic sites. McCreary, PharmD, BCIDP) appeared first on Rethinking Clinical Trials.

Rethinking Clinical Trials
JANUARY 31, 2024
The first step is to develop measures of success, the second step is designing the workflow, and the third step is evaluating pre-integration safety and effectiveness before a solution is put into clinical use. The next key decision point is clinical integration. For the CKD example, we combined data from Duke and claims data.

Rethinking Clinical Trials
NOVEMBER 21, 2022
Pragmatic Clinical Trials. In 2016, we published a pilot trial looking at high-flow nasal cannula (HFNC) therapy compared to CPAP in pediatric critical care. The step-up trial started recruitment in August 2019, 1,449 children were eligible, 600 children were randomized, and 595 consented. ? ? ? ? ? ? ? ?. Speakers. Key Points.

Rethinking Clinical Trials
FEBRUARY 9, 2023
The effectiveness of the intervention was then evaluated through a cluster randomized trial at four academic tertiary hospitals from December 21, 2020 to January 21, 2022. The study team developed the tool using high-quality evidence from randomized trials related to risk factors for subpopulations and guideline recommendations.

Rethinking Clinical Trials
AUGUST 30, 2023
The team collected focus group feedback from patients and clinicians and worked with IT professionals to pull data directly from the electronic health record and design an interface that could be used by all patients. The RA PRO dashboard was tested as part of a stepped wedge, cluster randomized trial at the clinician level.

XTalks
JUNE 28, 2023
The approval is restricted to ambulatory patients within this narrow age range due to uncertainty around its effectiveness in older children, which Sarepta hopes to clarify in a confirmatory trial. As such, the agency is requiring Sarepta to confirm the therapy’s clinical benefit as part of the confirmatory trial.

Rethinking Clinical Trials
MARCH 1, 2023
One network is the lead, but the trial will be available in all four U.S. We are working on inclusion and making sure all of our trials have buy in across the group. We got both Merck and Lily to work together in the Phase II trial, then the data from that trial that showed survival benefit led to the Phase III study.

XTalks
APRIL 18, 2024
Roche has received Breakthrough Device designation from the US Food and Drug Administration (FDA) for its Elecsys pTau217 early Alzheimer’s blood test. The Elecsys pTau217 assay is designed to detect amyloid pathology, a hallmark of Alzheimer’s disease, by measuring phosphorylated tau protein in human plasma.

Rethinking Clinical Trials
AUGUST 18, 2022
These terms and designations were intended to restrict rights. The Trial Innovation Network Recruitment Innovation Center aims to positively impact human health by improving participant enrollment and retention in multi-center clinical trials. Study design and approach should support diversity goals. New Ideas study.

Rethinking Clinical Trials
JUNE 7, 2023
The trial was designed to randomize 1,000 patients who had an On-X aortic valve replacement at least 3 months prior to randomization with either a standard dose of Apixaban (5mg) or continued warfarin (the standard of care). The trial took place at 64 sites, and randomized 863 patients. Learn more Read about the PROACT Xa study.

Rethinking Clinical Trials
MARCH 28, 2023
It has grown to be a major force for supporting pragmatic research, both real-world evidence research and supporting observational research and pragmatic clinical trials. As the network was launched and the first trial, ADAPTABLE, was getting started, we came to the conclusion that we needed large, generalizable studies that were efficient.

Rethinking Clinical Trials
JUNE 29, 2022
Real-world evidence (RWE) is clinical evidence derived from analysis of RWD regardless of study design. The post June 24, 2022: FDA Draft Guidance on Real-World Evidence (John Concato, MD, MS, MPH) appeared first on Rethinking Clinical Trials. and the FDA Draft Guidance for RWD/RWE. pctGR, @Collaboratory1.

Rethinking Clinical Trials
AUGUST 10, 2023
“Serious games” are games designed for a purpose beyond pure entertainment. They use motivation, game design, competition, curiosity, collaboration, challenges, media, and learning to accomplish something. This can be useful in the context of clinical trials. The important thing is to learn and adapt to the changes.

Rethinking Clinical Trials
OCTOBER 27, 2022
Having a tech person on the study design team to advise on which devices are secure will allow as many people as possible to participate. Beyond privacy: A deeper understanding of the internet is required to protect digital trial participants. You need to involve them from the design phase. Learn more. Discussion Themes.
pharmaphorum
APRIL 27, 2022
AstraZeneca and Daiichi Sankyo have claimed a fifth breakthrough designation from the FDA for Enhertu, shortly after showing the drug extended survival in patients with HER2-low metastatic breast cancer. Drugs given the designation are hastened through the development process by the FDA, which can grant them a faster six-month review.

Rethinking Clinical Trials
NOVEMBER 21, 2023
The idea was to engage the patient, providers, and healthcare system leaders to design, refine, and implement the pilot. More than 13,000 patients were considered eligible for the UH3 trial and more than 9,500 were randomized in the 4 arms, with about 2,300 patients in each of the arms. The study would then recalculate the gaps.

pharmaphorum
DECEMBER 23, 2021
Daiichi Sankyo has been granted breakthrough status by the FDA for patritumab deruxtecan, a HER3-targeted antibody-drug conjugate (ADC) in clinical trials for lung cancer. The post FDA gives Daiichi Sankyo’s HER3 drug a breakthrough tag appeared first on.

Rethinking Clinical Trials
AUGUST 3, 2023
Design stand-alone data visualizations because one thing that happens in a social media world is that the graphs and visualizations very quickly escape their containers. Tags #pctGR, @Collaboratory1 The post Grand Rounds July 28, 2023: How Can Researchers Fight Misinformation About Medicine? We need to find a way to tackle this.

pharmaphorum
AUGUST 21, 2022
In this product, bupropion is designed to increase levels of dextromethorphan in the blood and extend its half-life. Axsome’s drug was given a breakthrough designation from the FDA, reflecting the need for new treatment options in patients at increased risk of self harm or suicide, and was also given a priority review.

The Pharma Data
OCTOBER 9, 2021
Roche (SIX RO, ROG; OTCQX RHHBY) now posted that gantenerumab, ananti-amyloid beta antibody developed for subcutaneous administration, has been granted Improvement Rectifier Designation by theU.S. This designation for gantenerumab marks the 39th Improvement Rectifier Designation for Roche’s portfolio of pharmaceuticals.

XTalks
AUGUST 23, 2021
This is not a debate on approval,” said Stacie Weninger, PhD, president of the F-Prime Biomedical Research Initiative, in a focused topic session on the impact of drug approval on future clinical trials held on Tuesday July 27. Now that [aducanumab] is approved, how will this affect clinical trials going forward?”.

XTalks
FEBRUARY 8, 2024
Along with the hefty price tag for screen time that may not be perceived to be a worthwhile investment, and despite favoring advertising at other major events like the Grammys, pharma tends to stay somewhat clear of all the Super Bowl hoopla.

XTalks
FEBRUARY 27, 2024
Since the activity of TILs can be suppressed by tumor cells, TIL therapies are designed to bolster the number of TILs. Addressing the high price tag, Iovance’s commercial chief Jim Ziegler said, “Payers have expressed their appreciation for the value proposition for Amtagvi.” billion into this T-cell immunotherapy segment since 2013.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content