The dermatology market is embarking on tremendous growth and sponsors need to be ready. Over the last 12 months, dermatology-focused studies have increased by roughly 20%, with top focus areas including psoriasis, atopic dermatitis, and alopecia. Projections suggest the dermatology market will grow from its current value of $17B in 2023 to $50B by 2030.
This rapid growth brings new challenges. For example, dermatology therapeutics are becoming more complex with novel methods of administration. At the same time, the growing number of studies means increased competition. Efficiency and speed are critical. Sponsors must innovate in ways that increase endpoint predictability without adding to the burden on sites.
Standardizing rater qualification and training can solve many of the challenges that dermatology trials pose. When done correctly, it can mitigate the burden for both sponsors and sites, expand rater diversity by training less experienced raters, and improve industry accuracy, consistency, and objectivity.
Centralized rater qualification and training also provides an opportunity to meet growing dermatology demand and complexity. By centralizing rater qualification data, study teams, sponsors and CROs have immediate access to site resources, enabling teams to select and engage the best sites for a trial.
Consistency is essential, and a centralized, standardized approach means each rater’s qualifications are assessed in the same manner. This helps ensure sponsors and investigators have the data-driven insights to inform study design and protect clinical endpoints.
It also provides the opportunity to introduce new sites to research, harnessing the expertise of experienced raters and expanding the pool of potential sites. Centralizing qualification data allows the industry to further develop raters, engage new sites in research, and identify where experienced raters are in order to tap into that knowledge. Including more sites with less experience helps increase participant diversity.
Likewise, standardized training tailored to the rater’s experience and expertise increases consistency and accuracy.
Rater Training in Dermatology Trials
Rater training hasn’t been the norm in dermatology studies, but it should be. Any dermatology endpoint is highly subjective, possibly even more subjective than CNS endpoints, where rater training is more commonly used.
Moreover, it can’t be assumed that all raters have the necessary background and experience to jump into rating immediately or that there are enough experienced raters for all sites and all studies.
The solution is atiered approach to training based on the education and experience of the rater, with supplemental training provided for less experienced raters. This approach allows sites and studies to include raters with minimal experience. Then, training can be provided to get them up to speed to rate those scales just as well as the extremely experienced raters.
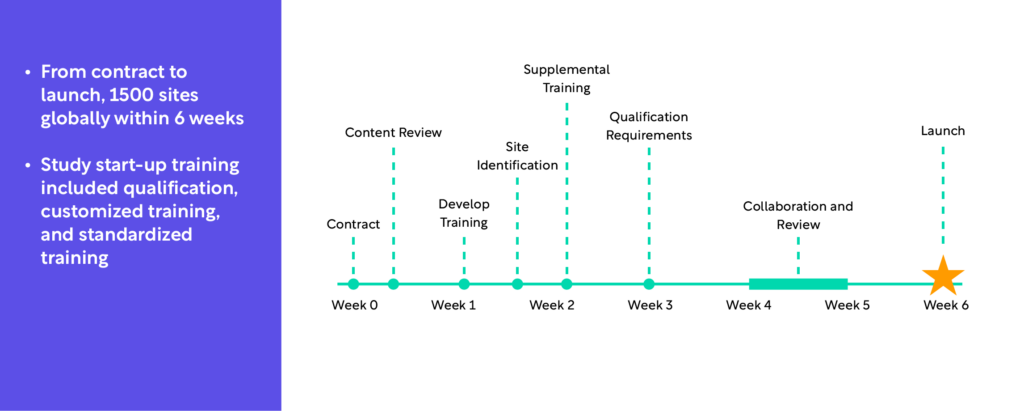
It’s important to note that raters can complete training quickly. For example, a top 20 pharmaceutical company applied this approach to a large global program with over 1,500 sites (Figure 1). From contract to launch, these 1,500 sites were ready within six weeks. Study start-up training included qualification, customized training, and standardized training.
Clinical Photography Improves Accuracy
Clinical photography provides another way to reduce subjectivity and enhance accuracy. Clinical photography allows raters to accurately assess change over time. It also supports using independent review to identify issues and provide an additional level of robust endpoint accuracy, eliminating as much bias as possible.
Strategies for Maximizing the Benefits of Photography
To maximize the benefits of photography, the first step is to proactively establish the image requirements. This involves setting photography parameters that are reproducible and consistent—and that meet the needs of the study and the site.
Proactively identifying the different parameters allows for the inclusion of any other data points the study team might be trying to acquire while the participant is in the clinic or practitioners are conducting various assessments. Ultimately, this enables the integration of additional potential endpoints in clinical biomarkers.
Next, you must adhere to core lab and imaging standards, such as de-identifying images to remove personal health information. These standards provide a level of quality control to ensure the highest quality images across the entire study.
Sponsors and CROs can integrate photography without adding to the site burden through a few recommended best practices:
- Integrate photography with eCOA whenever possible for a more integrated and consolidated approach.
- Ensure the photography solutions are easy to use.
- Provide comprehensive training for raters on photography, including how to acquire images to eliminate inconsistency across your sites.
Industry Moving Toward Standardized Training
The adoption of standardized training and endpoint training in all trials is growing at a rapid speed. In 2018, about 970 raters participated and trained on standardized training. In 2023, there are records of 25,000 raters accessing standardized training. It’s an exciting time for the dermatology market to adopt this approach to meet study challenges.
To learn more about standardizing rater qualification and training for your dermatology studies, watch WCG’s webinar, Optimizing Endpoint Assessments for Dermatology Studies.
Apply powerful expertise and technology to improve endpoint training for consistent outcomes
Contact us to learn how WCG’s one unified system for rater qualification and training mitigates the burden for both sponsors and sites, expands rater diversity, and improves endpoint accuracy, consistency, and objectivity.
