Will All Sites One Day be Completely Digital?
ACRP blog
MARCH 7, 2024
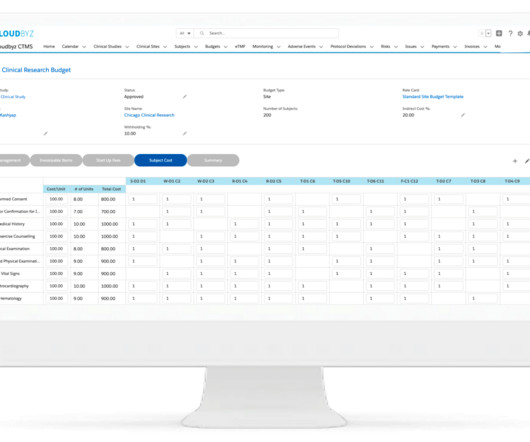
This is a sponsored message. It’s apparent that clinical trial sites are increasingly investing in technology to digitize and streamline their workflows. Despite this, many sites continue to rely on traditional paper-based methods or in-house digital documentation systems.



















































Let's personalize your content