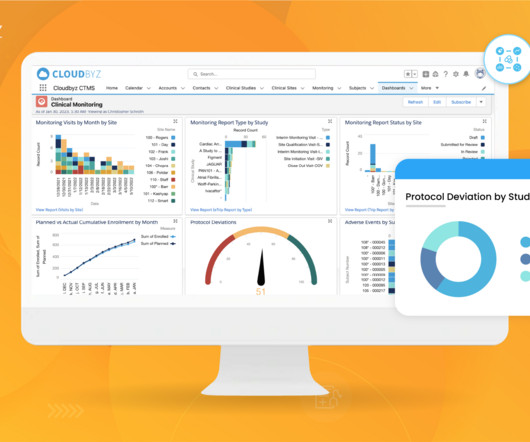
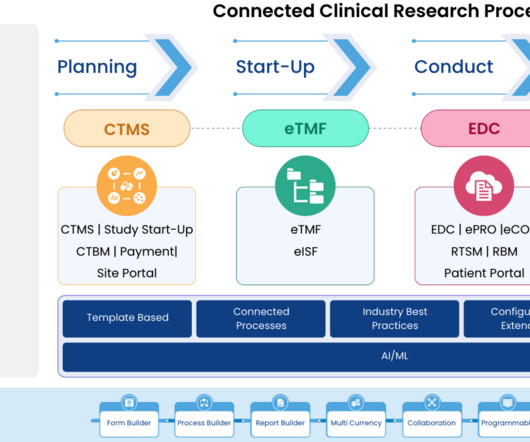
9 Ways Worldwide Optimizes Clinical Development for Your Biotech Growth Journey
Worldwide Clinical Trials
AUGUST 15, 2023
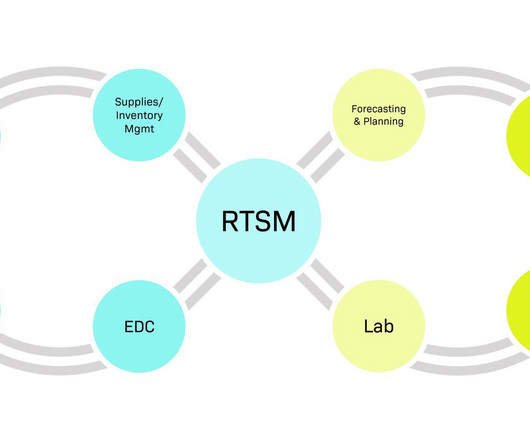
At Worldwide, we understand the significance of this collaboration and are dedicated to becoming an extension of your team, providing personalized clinical development solutions that align with your corporate objectives and milestones. Together, we drive study success and deliver results that matter to your stakeholders.















































Let's personalize your content