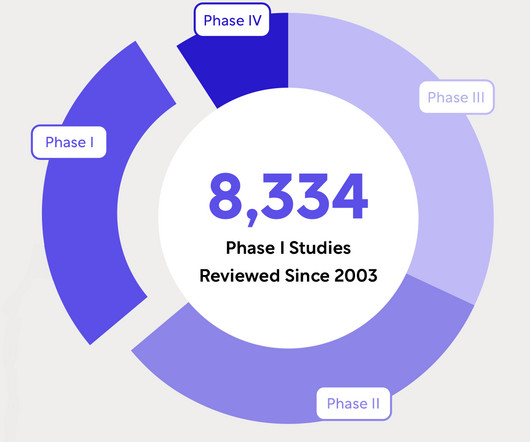
WCG Revolutionizes Ethical Review Process with IRB+ Service, Achieving Unprecedented Efficiency Gains
WCG Clinical
NOVEMBER 30, 2023
PRINCETON, NJ, November 30, 2023 – WCG, the global leader in providing solutions that measurably improve and accelerate clinical research, announced today the official launch of IRB+, a groundbreaking service that transforms the landscape of Institutional Review Board (IRB) operations.


































Let's personalize your content