Grand Rounds October 27, 2023: Digital, Decentralized and Democratized: Lessons From The Yale PaxLC Trial (Harlan M. Krumholz, MD, SM)
Rethinking Clinical Trials
OCTOBER 31, 2023
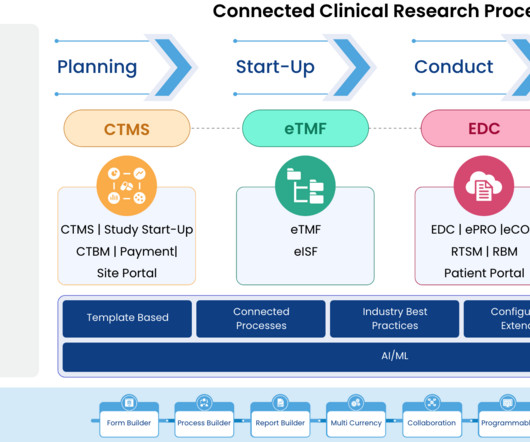
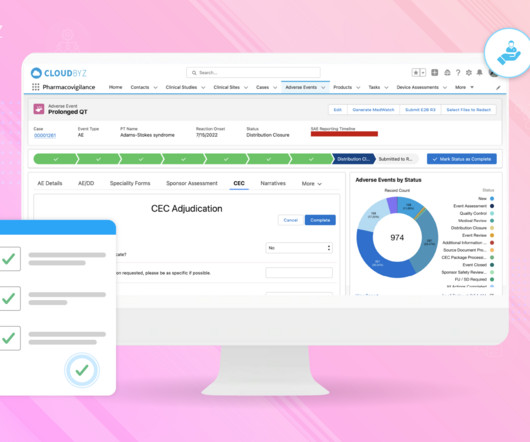
This talk describes the PaxLC Trial’s strategies to implement a digital, decentralized, and democratized approaches to multidisciplinary research that address the deficiencies of traditional research studies, which can tend to be hierarchical, siloed, slow, expensive, and inconvenient. We had to come up with solutions.



















































Let's personalize your content