Pharmacovigilance in Clinical Trials: Safeguarding Participant Safety and Ensuring Data Integrity
Cloudbyz
JULY 19, 2023
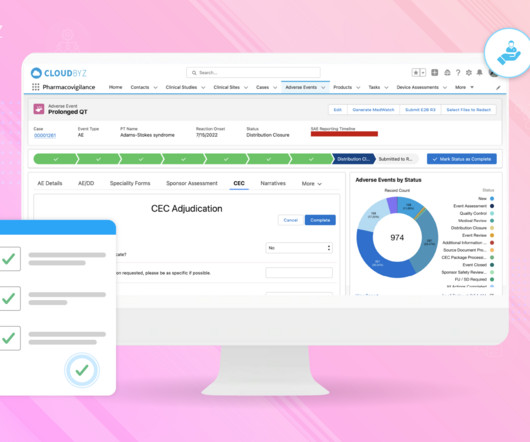
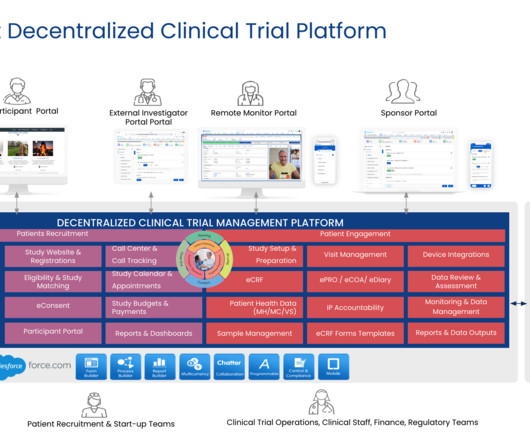
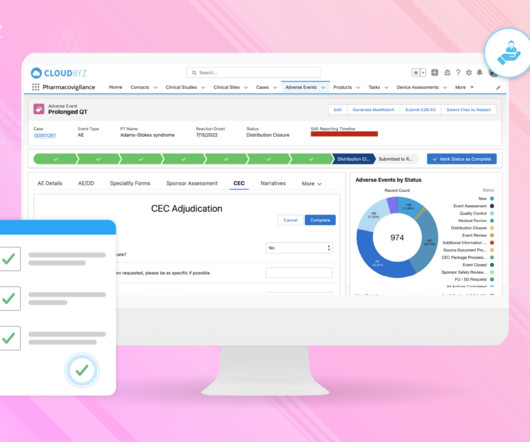
Pharmacovigilance plays a critical role in monitoring adverse events, collecting safety data, and reporting requirements to protect participants. This blog delves into the significance of pharmacovigilance in clinical trials, highlighting its role in participant safety, data integrity, and regulatory compliance.









































Let's personalize your content