FDA sets April verdict for Pfizer’s “underestimated” atopic dermatitis drug
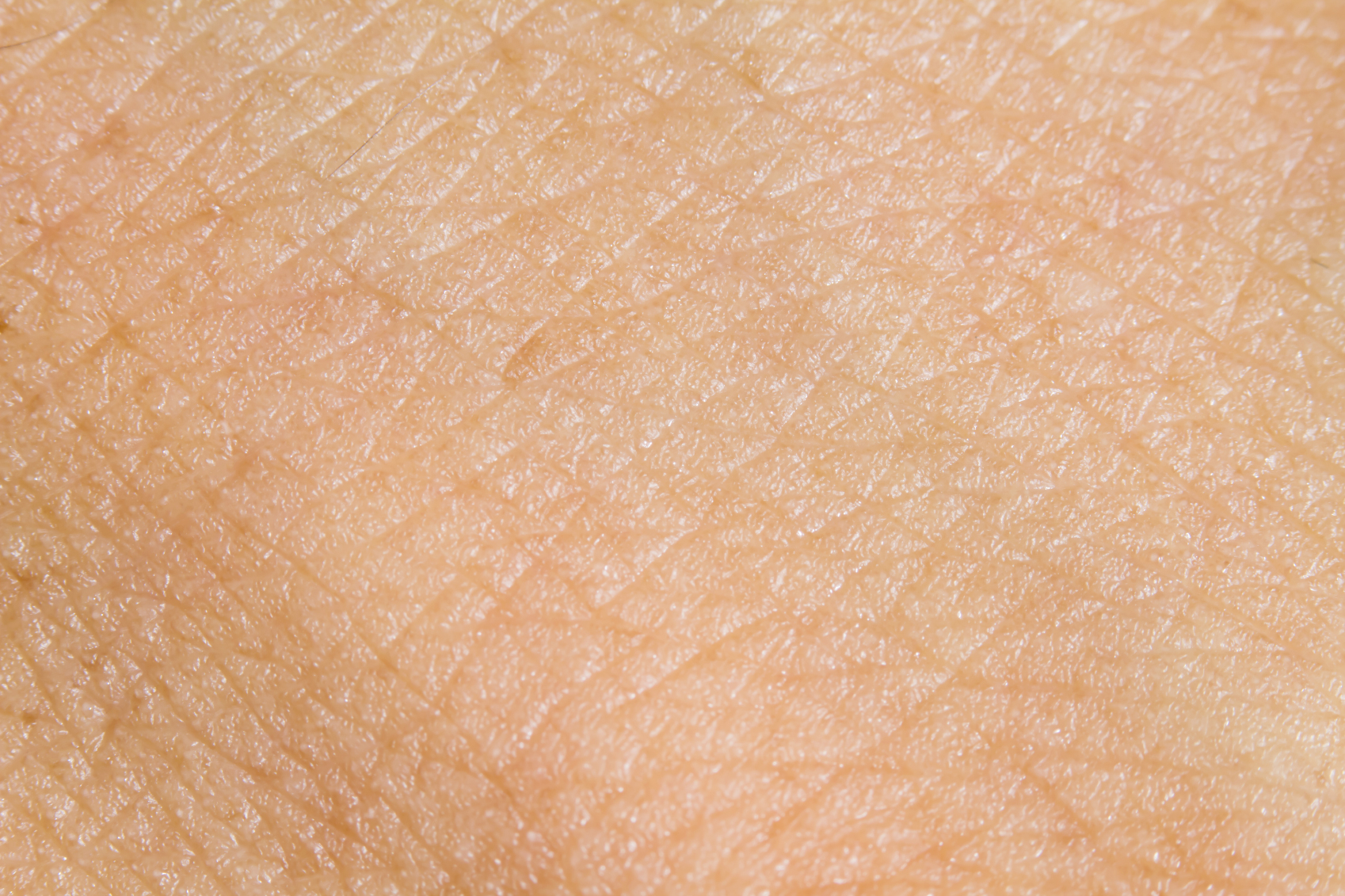
Pfizer could be just a few months away from getting FDA approval for its JAK1 inhibitor abrocitinib in atopic dermatitis, a drug that CEO Albert Bourla believes hasn’t been given the credit it is due by Wall Street analysts.
The US regulator has started a priority review of abrocitinib for mediate to severe atopic dermatitis in patients aged over 12, setting up a decision next April, while the EMA has also started a standard review that could lead to approval in the EU in the second half of 2021.
Bourla told analysts on Pfizer’s results call yesterday that abrocitinib is “the one potential near-term compound where we see the biggest difference compared with consensus.”
Pfizer sees abrocitinib and other JAK drugs coming through the pipeline for atopic dermatitis as expanding the number of patients getting treatment for the more severe end of the spectrum of symptoms – and it reckons that unlocks blockbuster sales potential.
“This is not a zero-sum game with the biologics in the treatment of moderate to severe atopic dermatitis,” said Bourla.
These patients currently rely on biologic drugs like Sanofi and Regeneron’s Dupixent (dupilumab), which dominates the category and saw sales rocket to more than $2 billion last year.
Sanofi sees plenty of additional upside, suggesting the drug could become a $10 billion brand at peak from expansion in atopic dermatitis as well as new indications like asthma, chronic rhinosinusitis with nasal polyps and eosinophilic oesophagitis.
Abrocitinib has previously been shown as effective in the JADE-MONO-1 and JADE-MONO-2 trials in subjects aged over 12, with a profile that looks like it could challenge its biologic rivals.
In particular, Pfizer says the drugs seem to have an impact on itch – often cited as the most bothersome symptom by atopic dermatitis patients.
Bourla and Angela Hwang – group president of Pfizer’s biopharmaceuticals division – think analysts are overlooking the sheer scale of atopic dermatitis as a condition with around 60 million sufferers aged over 12 worldwide.
They are also discounting abrocitinib’s profile, and the fact that almost two-thirds of patients treated with Dupixent don’t achieve clear or almost clear skin at 16 weeks, leaving room for improvement.
“Of those 60 million, only 7% of them today are being treated with a systemic agent,” said Hwang on the call. “So the systemic market opportunity has real potential to more than double with the introduction of better systemic treatment, because the patient need is just so high.”
There’s a precedent for that level impact for new biologic drugs such as interleukin inhibitors for psoriasis, which doubled the market over a 10-year period.
Claiming just 8% of the systemic atopic dermatitis treatment market would equate to $3 billion in abrocitinib sales, said Hwang.
Pfizer’s drug seems to have a lead in atopic dermatitis over other JAK inhibitors in the US, although Eli Lilly and Incyte's Olumiant (baricitnib) is closing on approval for the skin disorder in Europe, having picked up a CHMP positive recommendation last month. The partners plan to file for approval of Olumiant in the US and Japan this year.
AbbVie meanwhile has trials on the go for its fast-growing Rinvoq (upadacitinib) rival in this indication – including a head-to-head comparison with Dupixent due to read out in the coming months.












