Magnus Medical’s SAINT Neuromodulation System Gets FDA Clearance to Treat MDD
XTalks
SEPTEMBER 9, 2022
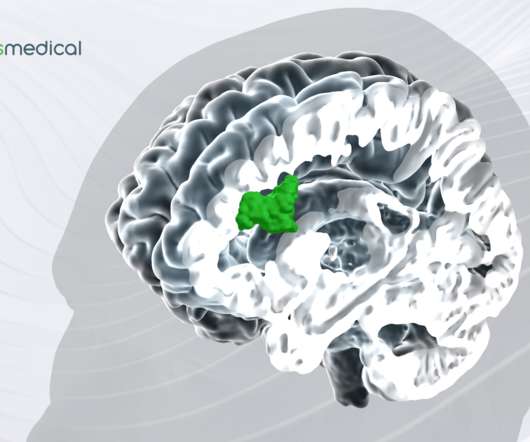
Magnus Medical received exclusive licensing rights to commercialize the SAINT (Stanford Accelerated Intelligent Neuromodulation Therapy) technology from Stanford University. Related: Xtalks Voices: Magnus Medical CEO Dr. Brett Wingeier Talks About Neurostimulation for Depression – Xtalks Life Science Podcast Ep.











Let's personalize your content