AstraZeneca and Nona Biosciences sign agreement for monoclonal antibody
Pharmaceutical Technology
MAY 23, 2024
AstraZeneca has entered into a worldwide licence and option agreement with Nona Biosciences for a preclinical monoclonal antibody.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
 Antibody Related Topics
Antibody Related Topics 
Pharmaceutical Technology
MAY 23, 2024
AstraZeneca has entered into a worldwide licence and option agreement with Nona Biosciences for a preclinical monoclonal antibody.

Pharmaceutical Technology
JULY 25, 2024
Dren Bio has entered a strategic partnership with Novartis Pharma to develop therapeutic bispecific antibodies to treat cancer.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
FEBRUARY 19, 2024
Almirall has signed a licensing agreement for the acquisition of worldwide rights to Novo Nordisk’s IL21-hindering antibody NN-8828.
Bio Pharma Dive
MAY 25, 2023
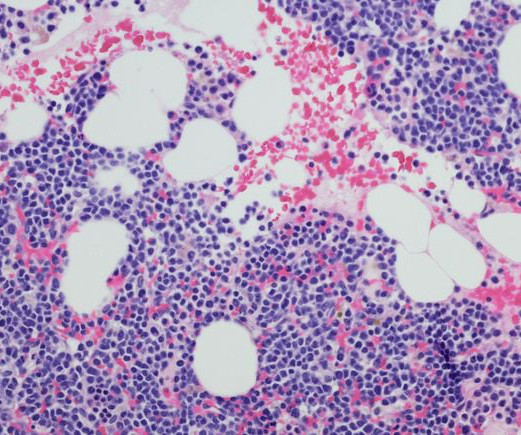
A pair of dual-acting antibodies displayed early potential to become a new type of drug regimen for the blood cancer, but led to a high rate of side effects as well.

Pharmaceutical Technology
MARCH 16, 2023
Talem Therapeutics, a subsidiary of Immunoprecise Antibodies, has entered a multi-target artificial intelligence (AI)-driven antibody discovery collaboration with Libera Bio. The MPN Technology provides an elegant way for delivering antibodies inside tumour cells.

Bio Pharma Dive
MAY 7, 2024
The $200 million round is the latest evidence that surging interest in autoimmune disease cell therapies could expand to include developers of bispecific antibodies, too.

Pharmaceutical Technology
MAY 2, 2024
Discover how Agenus Inc's groundbreaking patent for antibodies targeting TIM-3 is revolutionizing therapeutic antibody development. Explore the specific amino acid sequences and methods for efficient antibody production.

Pharmaceutical Technology
AUGUST 24, 2023
Japan has granted a new drug substance patent for BioArctic’s monoclonal antibody (mAb), BAN0805, to treat Parkinson’s disease.
Pharmaceutical Technology
FEBRUARY 15, 2023
In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Immuno-oncology: Leading companies in cancer monoclonal antibody therapy. Immatics is one of the most important players concerning innovation surrounding cancer monoclonal antibody therapy.

Pharmaceutical Technology
MARCH 7, 2024
Gilead Sciences has entered an agreement with Merus for the discovery of dual tumour-associated antigens targeting trispecific antibodies.

Pharmaceutical Technology
MAY 8, 2024
Zenas signed a licencing deal for Asia-Pacific territories for the bispecific antibody, obexelimab, with Bristol Myers Squibb in September.

Pharmaceutical Technology
JUNE 10, 2024
Discover Vir Biotechnology's groundbreaking patent for a monoclonal antibody targeting Staphylococcus aureus infections. Learn how this innovative composition binds to key proteins for potential treatment.

Pharmaceutical Technology
APRIL 4, 2024
Discover Xencor Inc's groundbreaking patent for novel GPC3 binding domains and bispecific antibodies targeting GPC3-associated cancers. Explore the innovative method for manufacturing these antibodies and their potential in cancer treatment.

Pharmaceutical Technology
OCTOBER 21, 2022
AbbVie has announced the acquisition of UK-based biotechnology firm DJS Antibodies for nearly $255m in cash at closing. DJS focuses on the discovery and development of antibody therapies that act on difficult-to-drug disease-causing proteins, such as G protein-coupled receptors (GPCRs).

Pharmaceutical Technology
MAY 28, 2024
J&J has made its second dermatology deal this month, adding Numab’s bispecific antibody NM26 to its growing atopic dermatitis drug portfolio.

Pharmaceutical Technology
JULY 21, 2022
AstraZeneca has signed a deal with the Federal Office of Public Health (FOPH) of Switzerland to deliver over 1,200 doses of antibody therapy, tixagevimab and cilgavimab combination (AZD7442), for Covid-19 prevention and treatment. In June 2020, these antibodies, discovered at Vanderbilt University Medical Center, were licensed to AstraZeneca.

Bio Pharma Dive
MAY 28, 2024
The deal is J&J’s second this month involving bispecific antibodies, which the company has prioritized to build its immune and cancer drug pipeline.

Pharmaceutical Technology
JANUARY 9, 2023
Eisai and Biogen have received approval for their antibody Leqembi (lecanemab-irmb) , 100mg/mL injection for intravenous use, from the US Food and Drug Administration (FDA) under the Accelerated Approval Pathway to treat Alzheimer’s disease (AD). The regulatory approval is based on the data obtained from the Phase II trial.

Pharmaceutical Technology
APRIL 30, 2024
Discover a groundbreaking patent for treating CLEC12A positive cancer with bispecific antibodies. Learn about a unique dosing strategy to target cancer cells effectively while sparing hemopoietic stem cells.

Pharmaceutical Technology
JUNE 10, 2024
Discover Kodiak Sciences Inc's innovative method for treating wet age-related macular degeneration with an anti-VEGF antibody conjugate. Patented approach offers extended therapeutic effects for at least 12 weeks, providing a less frequent treatment option.
Pharmaceutical Technology
MAY 8, 2023
In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Anti-influenza antibody compositions. However, not all innovations are equal and nor do they follow a constant upward trend.

Pharmaceutical Technology
DECEMBER 22, 2023
Ono has signed an agreement with EVQLV to utilise its AI-powered antibody design engine for the development of new antibody drugs.

Pharmaceutical Technology
APRIL 18, 2024
Discover how Vir Biotechnology's patented multispecific antibodies target distinct Zika virus epitopes, neutralizing ZIKV infection. Explore the innovative potential for prophylaxis and treatment in this groundbreaking patent.

Pharmaceutical Technology
APRIL 23, 2024
Discover how Yuhan Corp's patent for anti-PD-L1 IgG class antibodies revolutionizes manufacturing yields. Learn about the advancements in production efficiency and potential therapeutic applications.

Pharmaceutical Technology
APRIL 10, 2024
Discover the groundbreaking patent by Kyowa Kirin Co Ltd for a monoclonal antibody targeting CCR1, inhibiting its activation by CCL15. Explore the therapeutic and diagnostic potential for CCR1-related diseases.

Bio Pharma Dive
MAY 16, 2024
The deal is part of a plan by J&J to build a portfolio of “differentiated” bispecific antibodies, an increasingly popular area of drug development.

Pharmaceutical Technology
APRIL 9, 2024
The grant will support the preclinical development of the antibody acquired by Basilea from Spexis in January.
Pharmaceutical Technology
FEBRUARY 22, 2024
The company’s monoclonal antibody therapy, IO-202, has received its second orphan drug designation as a treatment for a blood cancer, CMML.

Pharmaceutical Technology
APRIL 22, 2024
Discover a broader repertoire of antibodies with a recently granted patent. Enhance immunoglobulin diversity with AbCellera's patented method for isolating B cells from genetically modified mice.

Pharmaceutical Technology
MARCH 28, 2024
Revolutionize cancer treatment with OSE Immunotherapeutics' patented anti-SIRPa antibodies targeting human SIRPa for enhanced T cell response and antigen presentation. Discover a comprehensive approach to treating various cancers with innovative combination therapies.

Pharmaceutical Technology
APRIL 23, 2024
UCB SA patent for IL-17A and IL-17F antibody molecules with expression vectors and host cell methods. Discover the innovative approach to efficient nucleic acid expression.
Bio Pharma Dive
JUNE 16, 2021
The biotech said it will seek an expanded FDA clearance after results from the RECOVERY trial showed its antibody can lower mortality among certain hospitalized COVID-19 patients.

Drug Discovery World
JUNE 24, 2024
This eBook explores the challenges and opportunities in the therapeutic antibody market, the range of applications for these therapies and the move towards more targeted and effective next-generation antibody treatments.

Pharmaceutical Technology
MAY 24, 2023
HBM’s wholly owned subsidiary Nona Biosciences has entered an agreement with OPKO Health’s ModeX Therapeutics for the discovery of antibodies. ModeX Therapeutics will gain access to the fully human Harbour Mice platforms of Nona Bioscience for integration into its MSTAR platform to expedite the monoclonal antibodies’ discovery.

Pharmaceutical Technology
APRIL 15, 2024
Discover Genzyme Corp's innovative method for detecting anti-drug antibodies in human body fluids. Learn how their patented process reduces interference in drug assays effectively.

Pharmaceutical Technology
FEBRUARY 22, 2024
Ono Pharmaceutical has partnered with EME to advance the drug discovery of VHH antibodies to develop new therapeutic agents.

Bio Pharma Dive
APRIL 21, 2023
A decision by the Supreme Court could have far-reaching effects on the lucrative market for antibody drugs, spurring responses from many of the industry’s top companies.

Pharmaceutical Technology
APRIL 1, 2024
Treat systemic lupus erythematosus effectively with UCB SA's patented method using a specific antibody to CD154. Significant improvements in SLE indices and outcomes. Explore the potential of this innovative treatment now!

BioPharma Reporter
JUNE 6, 2024
Alchemab Therapeutics, an antibody discovery company focused on identifying antibodies from individuals resilient to disease, has been awarded a grant of $595,000 by The Michael J. Fox Foundation for Parkinsonâs Research (MJFF) to propel its Parkinsonâs disease program forward.

Drug Discovery World
JUNE 25, 2024
A new eBook, sponsored by Sino Biological, is now available for download entitled ‘ The impact of therapeutic antibody discovery ’. The first monoclonal antibody (mAb), Orthoclone, was approved in 1986 to help prevent rejection in organ transplantation.

Pharmaceutical Technology
NOVEMBER 28, 2023
Boehringer Ingelheim has entered an agreement with IBM to leverage the latter’s foundation model technologies for antibody discovery.

BioSpace
MAY 23, 2024
Phase I/II data for Regeneron Pharmaceuticals’ costimulatory bispecific antibody were disappointing, with only one complete response when used as a combination treatment with Libtayo for solid tumors.

Bio Pharma Dive
JUNE 18, 2021
drugmaker is the latest to invest large sums of money in antibody-drug conjugates, following recent deals by Gilead, Merck and AstraZeneca.

Pharmaceutical Technology
SEPTEMBER 21, 2023
AbCellera and Regeneron have extended their current multi-target collaboration to discover therapeutic antibodies for up to eight targets
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content