FDA approves first gene therapy for hemophilia B
Bio Pharma Dive
NOVEMBER 22, 2022
The treatment, which is for the less common “B” form of the bleeding disorder, will be sold in the U.S. by maker CSL for $3.5 million.
Bio Pharma Dive
NOVEMBER 22, 2022
The treatment, which is for the less common “B” form of the bleeding disorder, will be sold in the U.S. by maker CSL for $3.5 million.
Pharmaceutical Technology
NOVEMBER 25, 2022
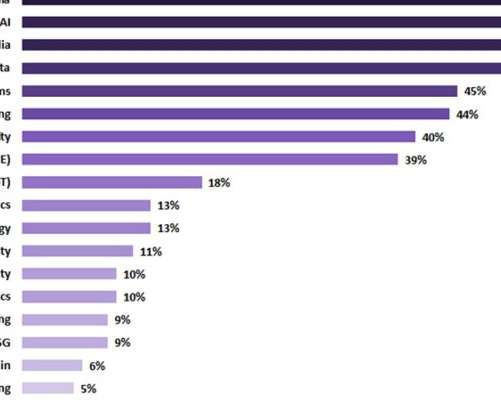
Artificial intelligence (AI) was seen as one of the top current investment priorities and was thought to continue to attract investment in the healthcare sector in the upcoming two years, according to GlobalData's latest report ‘Digital Transformation and Emerging Technology in the Healthcare Industry - 2022 Edition’. In this survey-based report tracker, digital media was prioritised as a top current investment target, with 53% of surveyed respondents confirming that their companies are currentl
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Bio Pharma Dive
MARCH 24, 2022
At least nine biotechs working in cell or gene therapy have announced layoffs, cost cuts or restructured their research since December — restructurings that have coincided with a stock market downturn.
Pharmaceutical Technology
DECEMBER 21, 2022
Artificial intelligence (AI) continued to stay in the news with several high-profile deals this year, as the pharmaceutical industry readily took to adopting AI models to improve drug discovery. But as the field grows in leaps and bounds, many authorities have prioritised the release of new guidelines, frameworks, and regulations to keep pace with these advances.
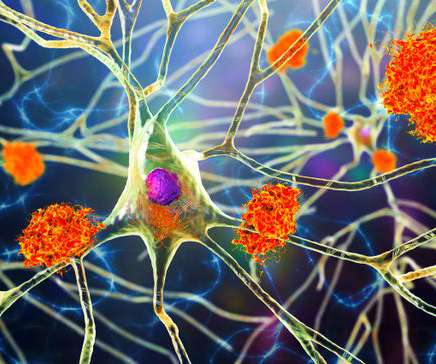
Bio Pharma Dive
NOVEMBER 29, 2022
At a medical conference, the companies detailed clinical trial results that could help support approval of their drug, lecanemab. However, some doctors aren’t yet convinced the medicine’s risks are worth its potential benefits.
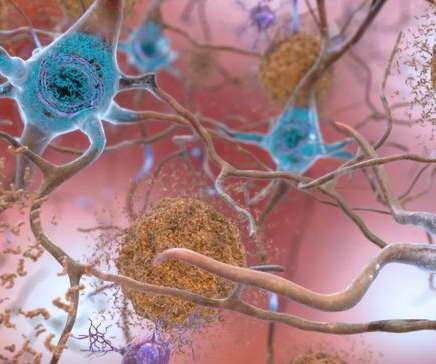
Bio Pharma Dive
SEPTEMBER 28, 2022
The companies said the drug, called lecanemab, met all of its goals in the Phase 3 study. The data are a significant finding and provide stronger support for a much-debated hypothesis for treating Alzheimer’s.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

STAT News
DECEMBER 20, 2022
Lots of people struggle to get enough sleep — and the responsibility for fixing the problem tends to fall on the individual. Experts offer advice like reducing screen time, exercising more, or just going to bed earlier in the evening. But many restless nights can’t be solved with blackout curtains, ear plugs, or other typical suggestions.
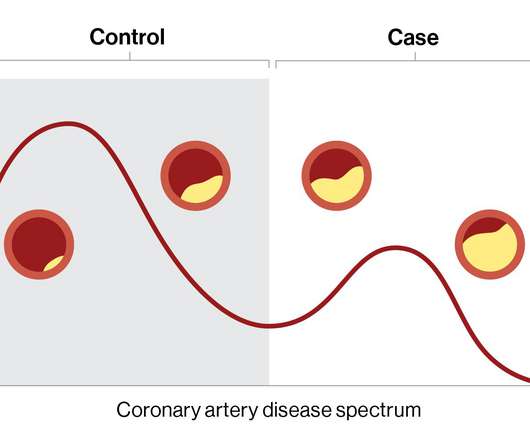
Medical Xpress
DECEMBER 20, 2022
Using machine learning and clinical data from electronic health records, researchers at the Icahn School of Medicine at Mount Sinai in New York constructed an in silico, or computer-derived, marker for coronary artery disease (CAD) to better measure clinically important characterizations of the disease.
BioPharma Reporter
NOVEMBER 22, 2022
GeNeuro has recruited the first patients in a Phase 2 trial evaluating temelimab against long-COVID: assessing the efficacy of the treatment for improving cognitive impairment and fatigue.
pharmaphorum
MAY 19, 2022
Moderna has joined forces with non-profit organisation IAVI on a third phase 1 trial of its candidate HIV vaccine in Africa, where the burden of the virus is still being keenly felt. IAVI (the International AIDS Vaccine Initiative) has started screening subjects to be included in the study, called IAVI G003, at centres in Rwanda and South Africa, said the biotech.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Outsourcing Pharma
JANUARY 12, 2022
The company plans to launch a Phase Ib/II study of its compound NVG-291 in collaboration with researchers at Northwestern Universityâs Shirley Ryan AbilityLab.

NY Times
JUNE 5, 2022
The study was small, and experts say it needs to be replicated. But for 18 people with rectal cancer, the outcome led to “happy tears.”.

Fierce Pharma
NOVEMBER 10, 2022
AstraZeneca withdraws US COVID vaccine application, shifts focus to antibody treatments. aliu. Thu, 11/10/2022 - 09:02.

Pharmaceutical Technology
NOVEMBER 14, 2022
In a field dominated by antibodies and small molecules, two cell-therapy based approaches have come under the spotlight for showing early signs of efficacy in treating lupus. In September, a group from Friedrich Alexander University Erlangen-Nuremberg reported that five patients with lupus achieved remission after an infusion of autologous chimeric antigen receptor (CAR)-T cells led to a deep depletion of B cells.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Bio Pharma Dive
SEPTEMBER 30, 2022
The drug, which will be sold as Relyvrio, showed modest benefits in function and survival in testing. It also became the latest test of the FDA's flexibility toward new therapies for neurological disorders.
Bio Pharma Dive
NOVEMBER 3, 2022
In a panel discussion hosted by BioPharma Dive, venture capitalists and CEOs discussed how startups can navigate a challenging market as well as possible ripple effects from the new U.S. drug pricing law.

Bio Pharma Dive
AUGUST 17, 2022
The regulator cleared the biotech’s medicine Zynteglo for transfusion-dependent beta thalassemia, giving patients a powerful new treatment option. But it will come at a very high cost of $2.8 million in the U.S.
Bio Pharma Dive
AUGUST 3, 2022
The success of Alnylam's treatment in a trial known as APOLLO-B could pave the way for the biotech to reach consistent profitability, should the FDA sign off on a planned approval application.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
Bio Pharma Dive
OCTOBER 18, 2022
At least five startups have emerged with new ways to genetically modify immune cells within the body, an approach that, if successful, could widen the field of CAR-T treatment.

Bio Pharma Dive
OCTOBER 18, 2022
The University of Pennsylvania immunologist and inventor of Kymriah spoke with BioPharma Dive about working with pharma, starting companies and the future of the cell therapy field.

Bio Pharma Dive
FEBRUARY 6, 2022
For the first time in years, biotechs no longer have an easy path onto Wall Street, a market reversal that could change what the next generation of young drugmakers looks like.

Bio Pharma Dive
JUNE 15, 2022
A closely watched study missed its goal, failing to prove the antiviral pill’s benefit in a broader population than the high-risk individuals for which it’s currently cleared.

Advertiser: FourKites
A research study conducted by The Journal of Commerce and FourKites surveyed hundreds of international shippers, exploring how their usage of global supply chain visibility technology has evolved since the onset of global disruptions caused by COVID-19. For international shippers, ocean freight visibility has evolved from optional to essential and satisfaction with visibility varies greatly depending on how it is obtained and delivered.
Bio Pharma Dive
OCTOBER 6, 2022
Nested Therapeutics touts a deep bench of scientific leaders and advisers, including Kevan Shokat, whose work drugging KRAS — a cancer-related gene once thought to be undruggable — helped lead to the development of Amgen’s Lumakras.

Bio Pharma Dive
NOVEMBER 8, 2022
Over the past several years, the investment firm has served as a financial adviser on biopharma acquisitions worth more than $200 billion in total, with the latest announced Monday.
Bio Pharma Dive
JANUARY 2, 2022
Biotech stocks ended 2021 in a slump. But positive results from eagerly anticipated studies in breast cancer, schizophrenia and Alzheimer's disease could help turn the sector's fortunes around.

Bio Pharma Dive
JANUARY 12, 2022
Analysts expect that deal engines are ready to start firing again following the recent quiet period. Still, uncertainty remains about how biopharma acquisitions could play out in the coming months.

Speaker: Steve Goldstein, Sales Leader
Are you currently in sales, or involved in a business that depends on strong sales results? What about the extremely competitive world of medical device sales? What are some of the top challenges your customers face and how do you approach understanding what’s most important to them? Join Steve Goldstein, Sales Success Coach, Motivational Speaker and Medical Device Sales Leader from Gold Selling LLC., to discover critical strategies and approaches you can take to engage your customers, achieve g

Bio Pharma Dive
JANUARY 10, 2022
Pfizer's work with mRNA vaccines led it explore other applications of the technology, resulting in a multi-year partnership with the high-profile biotech Beam Therapeutics on gene editing treatments for rare diseases.

Bio Pharma Dive
JANUARY 19, 2022
Hal Barron, a key figure in GSK's efforts to revitalize its drug research, will step down as its top scientist at a time when the company is feeling heat from investors to deliver faster growth.

Pharmaceutical Technology
AUGUST 4, 2022
Soaring patient numbers are stretching hospital capacity across the globe, forcing healthcare providers and their partners to think laterally about how to meet the demand without doubling their resources. Smarter diagnostic tools and more sophisticated remote- and self-care models will have an increasingly important role to play, as long as any advances are seen to deliver excellent outcomes and a better patient experience.
Bio Pharma Dive
SEPTEMBER 2, 2022
Preliminary results from a Phase 1 study show Sangamo’s treatment to be safe and suggest it is working as intended, leading the biotech to move into the trial’s next phase.

Advertiser: Aggregage
New vaccine mandates and testing policies will affect employers with more than 100 workers. Get Paycor’s free, customizable vaccination policy template to communicate critical details and new requirements to your employees. Get Paycor’s Template today!
Let's personalize your content