Novavax seeks FDA approval for updated Covid-19 vaccine
Pharmaceutical Technology
JUNE 17, 2024
1 version of its Covid-19 vaccine, NVX-CoV2705, for individuals aged 12 years and above. Novavax has sought US FDA approval for an updated JN.1
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
 Vaccination Related Topics
Vaccination Related Topics 
Pharmaceutical Technology
JUNE 17, 2024
1 version of its Covid-19 vaccine, NVX-CoV2705, for individuals aged 12 years and above. Novavax has sought US FDA approval for an updated JN.1
Pharmaceutical Technology
MAY 8, 2024
AstraZeneca has begun the global withdrawal of its Covid-19 vaccine Vaxzevria, citing a surplus of updated vaccines.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JUNE 25, 2024
1 Covid-19 vaccine, NVX-CoV2705, for people aged 12 years and above. Novavax has sought EMA approval for its updated JN.1

Pharmaceutical Technology
JUNE 3, 2024
Moderna has secured approval from the US Food and Drug Administration (FDA) for mRESVIA (respiratory syncytial virus vaccine).

Advertiser: Aggregage
New vaccine mandates and testing policies will affect employers with more than 100 workers. Get Paycor’s free, customizable vaccination policy template to communicate critical details and new requirements to your employees. Get Paycor’s Template today!

Pharmaceutical Technology
JULY 26, 2024
Mankind Pharma has announced a definitive agreement for the acquisition of Bharat Serums and Vaccines (BSV) for Rs136.3bn ($1.6bn).

Pharmaceutical Technology
JULY 25, 2024
The UK MHRA has authorised Pfizer-BioNTech’s adapted Comirnaty vaccine against the JN.1 1 Covid-19 subvariant for adults and children.

Pharmaceutical Technology
MAY 1, 2024
AstraZeneca has maintained that while the vaccine may, in “very rare” cases, cause TTS, the casual mechanism for this effect remains unknown.

Bio Pharma Dive
MAY 1, 2024
Sales of the shingles vaccine Shingrix and the RSV shot Arexvy helped fuel quarterly revenue totals that surpassed analyst expectations, though the company warned momentum could slow in the months ahead.
Pharmaceutical Technology
MAY 10, 2024
The FDA approved WestGene’s mRNA therapeutic cancer vaccine as mRNA cancer vaccine development rises in popularity.
Pharmaceutical Technology
FEBRUARY 12, 2024
vaccine targeting Omicron variant. The UK MHRA has granted approval for a variation in licence of Pfizer-BioNTech’s Comirnaty XBB.1.5

Pharmaceutical Technology
JANUARY 29, 2024
The rapid sequencing of the SARS-CoV-2 and the subsequent development of mRNA vaccines led to the authorisation of the first of these vaccines in late 2020.

Bio Pharma Dive
MARCH 1, 2024
The expert committee is discussing whether, for certain older adults, to make a universal recommendation for vaccination, rather than the current policy of “shared decisionmaking.”

Bio Pharma Dive
JULY 3, 2024
The mRNA specialist plans to eliminate 30% of its workforce as part of a restructuring that will prioritize “high-value” projects like its cancer vaccines.

Pharmaceutical Technology
MAY 15, 2024
The US agency rejected the expanded use of Dynavax’s vaccine for adults on haemodialysis, citing insufficient efficacy and safety data.

Bio Pharma Dive
APRIL 1, 2024
The development of cancer vaccines has provided some hope in the battle against cancer worldwide; however, there are still many challenges to overcome when developing these life-saving treatments.

Pharmaceutical Technology
MAY 9, 2024
Anixa Biosciences has expanded its partnership with Cleveland Clinic to develop additional vaccines for cancers.
Pharmaceutical Technology
MAY 11, 2023
Results published in Nature for a personalised pancreatic cancer vaccine that uses neoantigens from patients’ tumours have lent further support to early positive signals. The vaccine, developed by BioNTech, led to half of the patients with pancreatic cancer in the Phase I trial remaining cancer-free 18 months later.
Pharmaceutical Technology
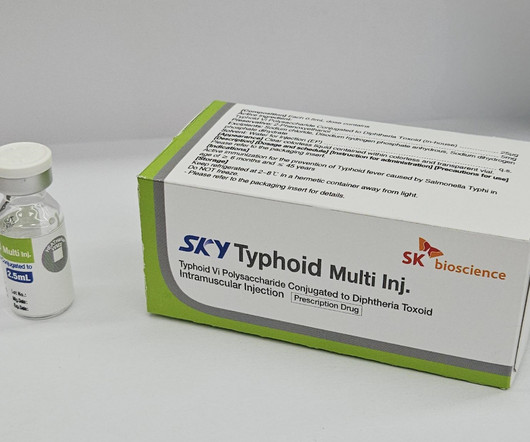
FEBRUARY 26, 2024
SK bioscience has achieved a significant milestone with its typhoid conjugate vaccine, SKYTyphoid, receiving WHO prequalification.

Bio Pharma Dive
OCTOBER 23, 2023
The clearance of the pentavalent shot Penbraya adds to Pfizer’s infectious disease portfolio as it adjusts to slumping COVID-19 vaccine sales.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 1, 2024
Scientists have developed a vaccine against a notorious drug-resistant superbug, targeting molecules on its surface that are also found on other bacteria and fungi.

Pharmaceutical Technology
MARCH 28, 2023
Following on from its Covid-19 vaccine programmes, BioNTech has set its sights on a range of infectious diseases for vaccine development. The company saw major successes with its Covid-19 vaccine, developed in collaboration with Pfizer. In response to the lower vaccine sales forecasts, BioNTech shares opened at 3.9%
Pharmaceutical Technology
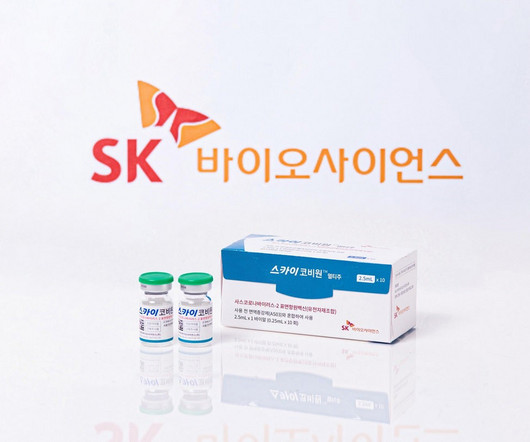
MAY 30, 2023
SK Bioscience has received marketing authorisation from the UK’s medicines and healthcare products regulatory agency (MHRA) for its Covid-19 vaccine, SKYCovion. The authorisation allows the distribution of the vaccine in Scotland, Wales and England.

Pharmaceutical Technology
MARCH 14, 2023
The National Health Surveillance Agency (ANVISA) in Brazil has granted approval for Takeda ’s dengue virus vaccine candidate, Qdenga (Dengue Tetravalent Vaccine [Live, Attenuated]) (TAK-003). The vaccine has received approval for preventing dengue disease in people aged four years to 60 years.

Pharmaceutical Technology
JULY 22, 2024
The company’s Tpoxx vaccine is used to treat orthopoxvirus but is only approved for treating smallpox by the US FDA.

Bio Pharma Dive
MAY 10, 2024
Sanofi will ally with the under-pressure biotech, paying $500 million upfront for rights to co-commercialize Novavax’s COVID shot and develop combination influenza vaccines.
Pharmaceutical Technology
FEBRUARY 7, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorisation for Takeda ’s dengue virus vaccine candidate, Qdenga (Dengue Tetravalent Vaccine [Live, Attenuated]). The vaccine candidate has been approved for active immunisation against the infection in people from four years of age.

Pharmaceutical Technology
JULY 3, 2024
Moderna has received a grant of $176m through the RRPV Consortium to expedite the development of mRNA-based pandemic influenza vaccines.

Pharmaceutical Technology
APRIL 14, 2023
Ghana’s Food and Drug Authority (FDA) has approved R21/Matrix-M malaria vaccine in children aged 5 to 36 months, marking the first regulatory clearance for the University of Oxford-developed vaccine in any country in the world. Children between the ages of five and 36 months are at highest risk of death from malaria.

Pharmaceutical Technology
MARCH 25, 2024
Cancer Research UK and the CRIS Cancer Foundation have awarded a £1.7m ($2.1m) grant for developing the lung cancer vaccine LungVax.
Pharmaceutical Technology
MAY 20, 2024
A recent study in England has shown that the HPV vaccine in teenagers reduced the rate of cervical cancer by up to 85%.

Pharmaceutical Technology
JUNE 24, 2024
The success of the Covid-19 messenger ribonucleic acid (mRNA) vaccines highlighted the major advantages of utilising mRNA technology in vaccine development.

Pharmaceutical Technology
JULY 15, 2024
Global vaccine coverage is yet to return to levels seen in 2019, shortly before the Covid-19 pandemic caused immunisation programme disruption.

Bio Pharma Dive
JUNE 27, 2024
The new guidance more strongly urges vaccination in adults who are older or at higher risk, but removes a prior recommendation for adults aged 60 to 74 years without certain risk factors.

Pharmaceutical Technology
JUNE 28, 2024
1-adapted Covid-19 vaccine. The EMA's CHMP has recommended granting marketing authorisation for Pfizer and BioNTech’s Omicron JN.1-adapted
Pharmaceutical Technology
APRIL 3, 2023
The World Health Organisation (WHO) has revised its recommendations regarding the use of Covid-19 vaccines following a meeting of the agency’s Strategic Advisory Group of Experts on Immunisation (SAGE). The panel emphasised that the extra booster dose recommendation only pertains to one shot and is not for continued annual booster vaccines.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 14, 2024
A Subject Expert Committee (SEC), which advices the national drug regulator on approvals and clinical trials related to Covid-19 vaccines, has recommended grant of permission to Serum Institute of India (SII) for Omicron XBB 1.5 The vaccine variant […]

Pharmaceutical Technology
JUNE 9, 2023
5+delta) protein vaccine (Sf9 cell). This marks the world’s first Covid-19 vaccine approved for emergency use against XBB descendent lineages of SARS-CoV-2. The vaccine has been developed by WestVac Biopharma along with the West China Medical Center and Sichuan University. recombinant Covid-19 trivalent (XBB.1.5+BA.5+delta)
Pharmaceutical Technology
NOVEMBER 29, 2023
Japan’s MHLW has approved CSL and Arcturus Therapeutics’ self-amplifying mRNA (sa-mRNA) Covid-19 vaccine, ARCT-154.
Bio Pharma Dive
JANUARY 23, 2023
Agency scientists are proposing to update COVID shots once a year to match circulating coronavirus strains, as well as simplifying current vaccination regimens.

Pharmaceutical Technology
JULY 3, 2024
GSK and CureVac have announced the restructuring of their existing partnership into a new licensing agreement for mRNA vaccine candidates.

Pharmaceutical Technology
NOVEMBER 17, 2023
in funding from the Bill & Melinda Gates Foundation for the bulk manufacturing of needle-free vaccines. Micron Biomedical has received $23.6m

Bio Pharma Dive
OCTOBER 13, 2023
The awards, to biotechs Gritstone, Codagenix and CastleVax, are part of the program’s plan to develop vaccines with broader or longer-lasting benefits.
Pharmaceutical Technology
OCTOBER 13, 2023
The Phase IIb SurVaxM vaccine study will enrol 265 patients and has an estimated completion date in Q2 2024.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content