Searching for True End to End Clinical Supply Visibility
Pharmaceutical Technology
NOVEMBER 9, 2023
By Iain Little, IRT Practice Lead at Tenthpin & Dan Silva Partner at Tenthpin With the method of managing the…
 Clinical Supply Related Topics
Clinical Supply Related Topics 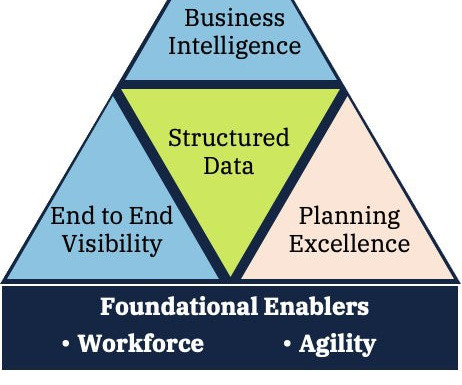
Pharmaceutical Technology
NOVEMBER 9, 2023
By Iain Little, IRT Practice Lead at Tenthpin & Dan Silva Partner at Tenthpin With the method of managing the…

Outsourcing Pharma
AUGUST 15, 2022
Signant Health has partnered with the software company o connect its platforms for the management of clinical supply chain inventory, labels, and content.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The Pharma Data
MAY 26, 2023
The expansion forms part of Catalent’s ongoing global strategy to increase its ability to handle, store and manage advanced therapies for clinical supply, and follows investments at its facilities in Philadelphia, Singapore, and Shanghai, China, in specialized, ultra-low temperature storage capabilities. With sites in the U.S.,

BioTech 365
OCTOBER 19, 2021
Clovis Oncology and ITM Announce Lutetium-177 Clinical Supply Agreement Clovis Oncology and ITM Announce Lutetium-177 Clinical Supply Agreement ITM to supply its medical radioisotope, no-carrier-added Lutetium-177, for the clinical development of Clovis Oncology’s Targeted Radionuclide Therapy candidate FAP-2286 BOULDER, … Continue reading (..)

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial.

Bio Pharma Dive
DECEMBER 7, 2020
Integrated CRO/CDMO solutions for accelerated and more efficient early drug development programs.

BioTech 365
NOVEMBER 23, 2021
MTTI and Monrol Have Signed a Clinical Supply Agreement for Lu-177 n.c.a. MTTI and Monrol Have Signed a Clinical Supply Agreement for Lu-177 n.c.a. WEST CHESTER, Pa. & & ISTANBUL–(BUSINESS WIRE)–#Lutetium–Eczac?ba??-Monrol Monrol Nuclear Products Co.

Advertisement
There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan.

Advertisement
When a CRO is bidding on a project where clinical supplies will be one of the aspects to manage on behalf of the client via a partner, leveraging the expertise of a chosen clinical supply partner can be a valuable resource in demonstrating the CRO’s understanding of and ability to deliver upon critical drug-supply related aspects of the project, and (..)
Let's personalize your content