Pharmaceutical waste containers and closure system
Pharmaceutical Technology
JUNE 4, 2024
Discover key considerations for selecting pharmaceutical waist containers. Find out about the latest technology used in pharmaceutical closure systems.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
 Containment Related Topics
Containment Related Topics 
Pharmaceutical Technology
JUNE 4, 2024
Discover key considerations for selecting pharmaceutical waist containers. Find out about the latest technology used in pharmaceutical closure systems.

pharmaphorum
MAY 9, 2024
How have drug container closure testing methods changed over time? This post explores the history and development of techniques to evaluate container integrity.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
NOVEMBER 29, 2023
GlobalData’s report assesses the drugs in the Bromodomain Containing Protein 4 pipeline by therapy areas, indications, stages, MoA, RoA, molecule type and the key players in the development pipeline.
Pharmaceutical Technology
OCTOBER 12, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has published updated guidance on valproate-containing medicines.

Pharmaceutical Technology
APRIL 17, 2024
Discover Eagle Pharmaceuticals Inc's patented stable bendamustine-containing composition for long-term storage. Learn about the sterile vial with antioxidants ensuring impurities less than 5% after 15 months.
Bio Pharma Dive
MAY 16, 2022
New mRNA technology: identifying the right drug containment solution is more important than ever.

Drug Patent Watch
MARCH 7, 2024
Buy Lactose-Free Medicines from Amazon This guide is designed to provide information for healthcare providers to… The post LACTOSE-Free Medicines: Which Drugs Contain LACTOSE? LACTOSE-Free Medicines, 2024 is part of DrugPatentWatch’s deep library of business intelligence on biopharmaceutical drugs.

Drug Patent Watch
MARCH 6, 2024
Buy Gelatin-Free Medicines on Amazon This guide is designed to provide information for healthcare providers to… The post GELATIN-Free Medicines: Which Drugs Contain GELATIN? GELATIN-Free Medicines, 2024 is part of DrugPatentWatch’s deep library of business intelligence on biopharmaceutical drugs.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 7, 2022
With an aim to improve the quality of drugs sold in the country, the Union health and family welfare ministry has released 9th edition of Indian Pharmacopoeia (IP) 2022 containing 92 new monographs for drugs, 12 new general chapters, 1,245 monographs for formulations, 930 monographs for active pharmaceutical ingredients (APIs) as well as dissolution (..)

Bio Pharma Dive
MARCH 14, 2023
“A generation of founders is now scarred,” one biotech CEO said, as small drugmakers grapple with the longer-lasting effects of SVB’s stunning collapse.
Drug Patent Watch
APRIL 13, 2023
Annual Drug Patent Expirations for ZOSYN+IN+PLASTIC+CONTAINER Zosyn In Plastic Container is a drug marketed by Wyeth Pharms and is included in one NDA. There… The post New patent expiration for Wyeth Pharms drug ZOSYN IN PLASTIC CONTAINER appeared first on DrugPatentWatch - Make Better Decisions.

NPR Health - Shots
JULY 25, 2022
Researchers advise that the coming days and weeks will be crucial as to whether the outbreak can be contained. Cases in the U.S. are 10 times higher than they were a month ago. Image credit: Kena Betancur/AFP via Getty Images)

Pharmaceutical Technology
JUNE 11, 2024
Discover how Amphastar Pharmaceuticals' patented medication delivery system prevents premature discharge during storage. Secure, moisture-controlled storage for medication devices.

Pharmaceutical Technology
MARCH 22, 2024
Discover Ironwood Pharmaceuticals' innovative patent for delayed release pharmaceutical compositions with linaclotide. Learn about the unique design and targeted therapeutic effects for gastrointestinal disorders.

Roots Analysis
FEBRUARY 28, 2022
The defects in the packaging of container closure system could adversely affect the sterility and stability of the drug through contamination via reactive gases, humidity and microbial ingress. So as to ensure the safety of drug and prevent any chance of risk, container closure integrity testing is performed on container closure systems.

AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 17, 2024
A new drug called kush is wreaking havoc in west Africa, particularly in Sierra Leone where it is estimated to kill around a dozen people each week and hospitalise thousands. The drug, taken mostly by men aged 18 to 25, causes people to fall asleep while walking, to fall over, to bang their heads against […]

Drug Discovery World
AUGUST 4, 2022
It may seem counterintuitive to spend time and money on planning for containment and delivery systems for a drug in the earliest stages of discovery when the chances of that molecule making it to market are still quite low. But waiting too long to assess containment and delivery carries its own risks. Testing compatibility.

Bio Pharma Dive
JULY 8, 2024
Myricx Bio is developing a type of payload that it thinks could work in tumors resistant to the toxins contained in ADCs like Enhertu and Trodelvy.
Drug Patent Watch
FEBRUARY 9, 2023
25+DEXTROSE+IN+PLASTIC+CONTAINER Cardene In 4.8% Dextrose In Plastic Container is a drug marketed by Chiesi and is included in one NDA. DEXTROSE IN PLASTIC CONTAINER appeared first on DrugPatentWatch - Make Better Decisions. Annual Drug Patent Expirations for CARDENE+IN+4.8%25+DEXTROSE+IN+PLASTIC+CONTAINER
Drug Patent Watch
FEBRUARY 9, 2023
25+DEXTROSE+IN+PLASTIC+CONTAINER Cardene In 5.0% Dextrose In Plastic Container is a drug marketed by Chiesi and is included in one NDA. DEXTROSE IN PLASTIC CONTAINER appeared first on DrugPatentWatch - Make Better Decisions. Annual Drug Patent Expirations for CARDENE+IN+5.0%25+DEXTROSE+IN+PLASTIC+CONTAINER
Drug Patent Watch
FEBRUARY 9, 2023
25+SODIUM+CHLORIDE+IN+PLASTIC+CONTAINER Cardene In 0.86% Sodium Chloride In Plastic Container is a drug marketed by Chiesi and is included in one NDA. It is available from… The post New patent for Chiesi drug CARDENE IN 0.86% SODIUM CHLORIDE IN PLASTIC CONTAINER appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
FEBRUARY 9, 2023
25+SODIUM+CHLORIDE+IN+PLASTIC+CONTAINER Cardene In 0.83% Sodium Chloride In Plastic Container is a drug marketed by Chiesi and is included in one NDA. It is available from… The post New patent for Chiesi drug CARDENE IN 0.83% SODIUM CHLORIDE IN PLASTIC CONTAINER appeared first on DrugPatentWatch - Make Better Decisions.

STAT News
JULY 26, 2022
WASHINGTON – Nearly three quarters of Americans give the pharmaceutical industry credit for helping contain Covid-19 — and for a sector that’s been roundly criticized for nearly a decade, that’s a reason to celebrate.

Medical Xpress
FEBRUARY 2, 2023
A UCLA-led study provides the first scientific evidence that brick and mortar pharmacies in northern Mexican tourist towns are selling counterfeit pills containing fentanyl, heroin, and methamphetamine. These pills are sold mainly to US tourists, and are often passed off as controlled substances such as Oxycodone, Percocet, and Adderall.

Medical Xpress
MARCH 13, 2023
A new study in the Journal of the Academy of Nutrition and Dietetics has determined that 60% of foods purchased by Americans contain technical food additives including coloring or flavoring agents, preservatives, and sweeteners. This represents a 10% increase since 2001. in 2001 to 4.5 in 2019.

Medical Xpress
APRIL 27, 2023
The role of sugars in public health continues to be urgently debated among nutrition scientists and health professionals—yet the science behind the effects of various fructose-containing sugars (e.g., sucrose/table sugar, high-fructose corn syrup, fructose/fruit sugar) on overweight and obesity has been unclear.

Pharma Times
FEBRUARY 12, 2024
The platform delivers digitalised patient data to improve clinical trials and development

Drug Patent Watch
APRIL 13, 2023
Annual Drug Patent Expirations for ZOSYN+IN+PLASTIC+CONTAINER Zosyn In Plastic Container is a drug marketed by Wyeth Pharms and is included in one NDA. There… The post New patent expiration for Wyeth Pharms drug ZOSYN IN PLASTIC CONTAINER appeared first on DrugPatentWatch - Make Better Decisions.

Medical Xpress
MAY 16, 2023
A vegan diet does not affect maternal breastmilk concentrations of vitamin B2 and carnitine, nutrients essential for the developing infant. These are the results of an Amsterdam UMC study, presented today at the 55th Annual Meeting of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).
Outsourcing Pharma
JANUARY 30, 2024
Two new offerings have been unveiled by drug delivery and solutions provider, Stevanto Group S.p.A.
Drug Patent Watch
JULY 11, 2021
Annual Drug Patent Expirations for BREVIBLOC+IN+PLASTIC+CONTAINER Brevibloc In Plastic Container is a drug marketed by Baxter Hlthcare and is included in one NDA. The post New patent expiration for Baxter Hlthcare drug BREVIBLOC IN PLASTIC CONTAINER appeared first on DrugPatentWatch - Make Better Decisions.

BioSpace
MAY 11, 2021
Nearly 50 infants with "bubble baby" disease developed a working immune system after they received a gene therapy that contained the AIDS virus, according to a new study.

XTalks
NOVEMBER 21, 2023
This episode features an interview with Attorney Temitope (Tope) Leyimu, a toxic exposure attorney at Motley Rice who is leading a lawsuit over hair relaxing products that contain harmful chemicals. The FDA recently issued a recommendation for the recall of chemical hair relaxers containing harmful chemicals.

Scienmag
MARCH 25, 2022
An orally absorbed tablet containing cannabidiol (CBD) effectively reduces pain after shoulder surgery with no safety concerns, a new study finds. Led by researchers in the Department of Orthopedic Surgery at NYU Langone Health, the study found that the tablet ORAVEXXTM safely managed pain after minimally invasive rotator cuff surgery, and did not (..)

STAT News
JANUARY 9, 2023
SAN FRANCISCO – U.S. Food and Drug Administration commissioner Robert Califf doesn’t disagree with the basic findings of a congressional investigation into the agency’s role in the controversial approval of Aduhelm, Biogen’s first Alzheimer’s drug. He just wishes the report’s tone had been different.

Medical Xpress
APRIL 2, 2023
Weeks after massive Cyclone Freddy hit Mozambique for a second time, the still-flooded country is facing a spiraling cholera outbreak that threatens to add to the devastation.

BioTech 365
SEPTEMBER 10, 2020
| Añadir a lista de seguimiento Biotech365 : MERCK MILLIPORE ANTISTATIC GROUNDING DEVICE FOR SOLVENT CONTAINERS BioMarketplace You want to propose your products or a Biotech … Continue reading →
Pharma Times
AUGUST 15, 2022
Study results show the candidate has demonstrated significantly higher antibodies against Omicron

Scienmag
MAY 4, 2022
Some items contain potentially harmful per- and polyfluoroalkyl substances (PFAS) to accomplish this feat, but companies aren’t required to disclose these “forever chemicals” on labels. Seems like kids are always getting into something, so products marketed toward them often claim to repel liquids.
Drug Patent Watch
JULY 11, 2021
Annual Drug Patent Expirations for BREVIBLOC+DOUBLE+STRENGTH+IN+PLASTIC+CONTAINER Brevibloc Double Strength In Plastic Container is a drug marketed by Baxter Hlthcare and is included in one NDA. It is available from three….
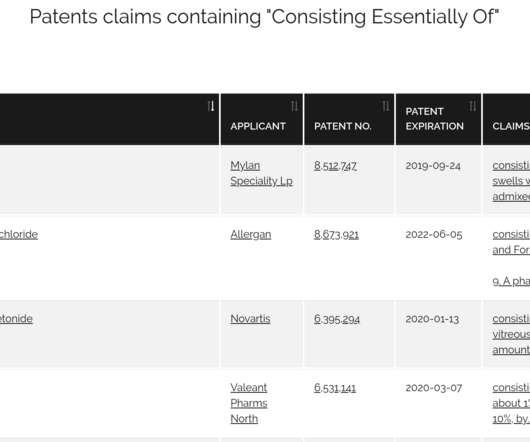
Drug Patent Watch
FEBRUARY 25, 2020
Court of Appeals for the Federal Circuit (CAFC) denied a petition to rehear arguments regarding the invalidity of patent claims containing the phrase…. The post 244 Drug Patents With Claims Containing “consisting essentially of” may be Unenforceable appeared first on DrugPatentWatch - Make Better Decisions.
Pharmaceutical Technology
APRIL 22, 2024
Health Canada has approved Merck’s KEYTRUDA (pembrolizumab), an anti-programmed cell death protein 1 (PD-1) therapy for use in combination with fluoropyrimidine- and platinum-containing-chemotherapy as a first-line treatment for adult gastric cancer patients.
Drug Patent Watch
JANUARY 11, 2021
Annual Drug Patent Expirations for ESMOLOL+HYDROCHLORIDE+IN+PLASTIC+CONTAINER Esmolol Hydrochloride In Plastic Container is a drug marketed by Hq Spclt Pharma and is included in one NDA. It is available from one….
BioPharma Reporter
NOVEMBER 24, 2020
Up to 10% of vaccines can be reportedly lost in transit due to breakage or fluctuations in sub-zero temperatures required for preservation, but a manufacturer of data-driven temperature-controlled smart containers says it can cut that rate to just 0.1%.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content