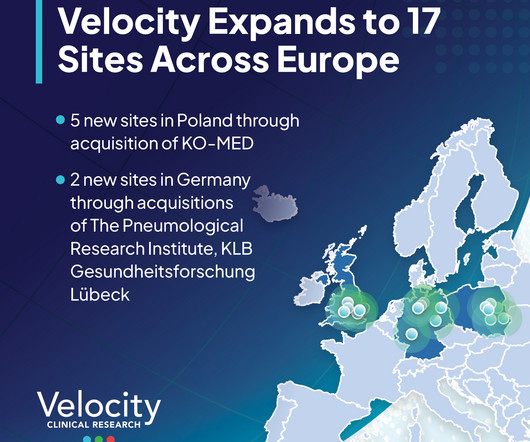
Velocity Expands to 17 Sites in Europe
Velocity Clinical Research
JANUARY 3, 2024
The acquisition of KO-MED is Velocity’s first foray into Oncology research, which accounts for roughly 40-50% of clinical trials globally. In most cases, the participant of a clinical trial benefits from taking part, which is of great importance, especially in oncological trials. and Europe.













Let's personalize your content