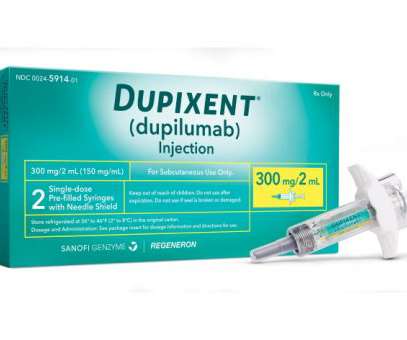
FDA clears Dupixent as first drug for rare skin disorder
pharmaphorum
SEPTEMBER 29, 2022
The disorder – driven by type 2 inflammation characterised by an antibody-based immune response – causes hard lumps or nodules to form on the skin that are so itchy they can lead patients to scratch themselves to the point of bleeding or pain. The post FDA clears Dupixent as first drug for rare skin disorder appeared first on.









Let's personalize your content